Current HIV Research ( IF 1 ) Pub Date : 2021-11-30 , DOI: 10.2174/1570162x19666210915102230 Santosh Mokale 1 , Deepak Lokwani 2 , Abdul Mujaheed 1
|
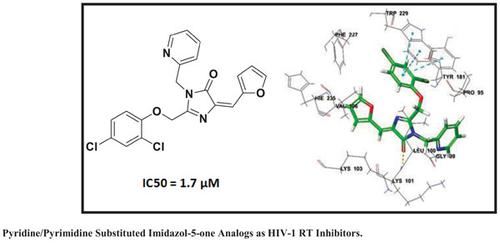
Background: This paper reports the synthesis, Non-nucleoside reverse transcriptase inhibitory (NNRTIs) activity and computational studies of 2-((4-chloro-2-subtitutedphenoxy) methyl)-4-(furan-2-ylmethylene)-1-substituted Pyridine/-pyrimidine-1H-imidazol-5(4H)-ones.
Methods: The imidazol-5-one analogs were synthesized by conventional method and characterized by FT-IR, NMR and mass spectral data. All compounds were evaluated for in-vitro NNRTI activity by using reverse transcriptase (RT) assay kit (Roche). The in-silico docking studies were conducted on RT enzyme to investigate binding site interactions of synthesized compounds. The MMGBSA method was also used to calculate the binding free energy between the inhibitors and RT enzyme. The MD simulation was further performed for the apo form of the RT enzyme and docked complex of compound A6-RT enzyme to better understand the stability of the protein-ligand complex.
Results: The bioactivity analysis revealed that most of the synthesized compounds showed significant inhibitory activity against RT enzyme and the IC50 value was found to be in the range of 1.76-3.88 μM. The computational studies suggest that the docked compounds form the H-bonding with amino acid residue Lys101 and hydrophobic interactions with amino acid residues Tyr188, Tyr181, Trp229, and Tyr318, which act as the primary driving forces for protein-ligand interaction.
Conclusion: The reported imidazol-5-one analogs can act as lead for further development of prospective RT inhibitors.
中文翻译:

吡啶/嘧啶取代咪唑 5-one 类似物作为 HIV-1 RT 抑制剂:设计、合成、对接和分子动力学模拟研究
背景:本文报道了 2-((4-氯-2-取代苯氧基)甲基)-4-(呋喃-2-基亚甲基)-1-取代的合成、非核苷类逆转录酶抑制(NNRTIs)活性和计算研究吡啶/-嘧啶-1H-咪唑-5(4H)-酮。
方法:采用常规方法合成咪唑-5-酮类似物,并通过FT-IR、NMR和质谱数据对其进行表征。通过使用逆转录酶(RT)测定试剂盒(Roche)评估所有化合物的体外NNRTI活性。对 RT 酶进行了计算机内对接研究,以研究合成化合物的结合位点相互作用。MMGBSA 方法也用于计算抑制剂和 RT 酶之间的结合自由能。进一步对RT酶的apo形式和化合物A6-RT酶的对接复合物进行MD模拟,以更好地了解蛋白质-配体复合物的稳定性。
结果:生物活性分析表明,大多数合成的化合物对RT酶具有显着的抑制活性,IC50值在1.76-3.88 μM范围内。计算研究表明,对接的化合物与氨基酸残基 Lys101 形成 H 键,并与氨基酸残基 Tyr188、Tyr181、Trp229 和 Tyr318 形成疏水相互作用,这是蛋白质-配体相互作用的主要驱动力。
结论:所报道的imidazol-5-one类似物可以作为进一步开发前瞻性RT抑制剂的先导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号