Chinese Journal of Catalysis ( IF 16.5 ) Pub Date : 2021-09-15 , DOI: 10.1016/s1872-2067(21)63804-4 Basil Sabri Rawah 1, 2 , Wenzhen Li 1
|
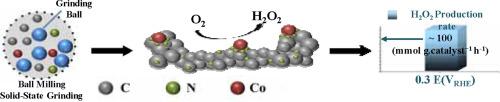
Electrocatalytic reduction of oxygen is a growing synthetic technique for the sustainable production of hydrogen peroxide (H2O2). The current challenges concern seeking low-cost, highly active, and selective electrocatalysts. Cobalt-nitrogen-doped carbon containing catalytically active cobalt-nitrogen (Co-Nx) sites is an emerging class of materials that can promote the electrochemical generation of H2O2. Here, we report a straightforward method for the preparation of cobalt-nitrogen-doped carbon composed of a number of Co-Nx moieties using low-energy dry-state ball milling, followed by controlled pyrolysis. This scalable method uses inexpensive materials containing cobalt acetate, 2-methylimidazole, and Ketjenblack EC-600JD as the metal, nitrogen, and carbon precursors, respectively. Electrochemical measurements in an acidic medium show the present material exhibits a significant increase in the oxygen reduction reaction current density, accompanied by shifting the onset potential into the positive direction. The current catalyst has also demonstrated an approximate 90 % selectivity towards H2O2 across a wide range of potential. The H2O2 production rate, as measured by H2O2 bulk electrolysis, has reached 100 mmol gcat.−1 h−1 with high H2O2 faradaic efficiency close to 85% (for 2 h at 0.3 V vs. RHE). Lastly, the catalyst durability has been tested (for 6 h at 0.3 V vs. RHE). The catalyst has shown relatively consistent performance, while the overall faradic efficiency reaches approximate 85% throughout the test cycle indicating the promising catalyst durability for practical applications. The formed Co-Nx moieties, along with other parameters, including the acidic environment and the applied potential, likely are the primary reasons for such high activity and selectivity to H2O2 production.
中文翻译:

嵌入氮掺杂碳的钴纳米颗粒电催化生成过氧化氢
氧的电催化还原是一种不断发展的合成技术,用于可持续生产过氧化氢 (H 2 O 2 )。当前的挑战涉及寻求低成本、高活性和选择性的电催化剂。含有催化活性钴氮 (Co-N x ) 位点的钴氮掺杂碳是一类新兴的材料,可以促进 H 2 O 2的电化学生成。在这里,我们报告了一种制备由许多 Co-N x组成的钴氮掺杂碳的简单方法。部分使用低能干态球磨,然后进行受控热解。这种可扩展的方法使用包含醋酸钴、2-甲基咪唑和 Ketjenblack EC-600JD 的廉价材料分别作为金属、氮和碳前体。在酸性介质中的电化学测量表明,本材料表现出氧还原反应电流密度的显着增加,伴随着起始电位向正方向移动。目前的催化剂还展示了在广泛的电位范围内对 H 2 O 2的大约 90% 的选择性。H 2 O 2生成速率,由 H 2 O 2测量大量电解,已达到 100 mmol g cat。-1 h -1具有接近 85% 的高 H 2 O 2法拉第效率(在 0.3 V与RHE 下持续 2 小时)。最后,对催化剂的耐久性进行了测试(在 0.3 V与RHE 下持续 6 小时)。该催化剂表现出相对一致的性能,而在整个测试循环中,整体法拉第效率达到约 85%,表明催化剂在实际应用中具有良好的耐久性。形成的 Co-N x部分以及其他参数,包括酸性环境和施加的电位,可能是对 H 2 O具有如此高的活性和选择性的主要原因2生产。


























 京公网安备 11010802027423号
京公网安备 11010802027423号