Chemosphere ( IF 8.8 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.chemosphere.2021.132266 Seung Yeop Lee 1 , Hyo Jin Seo 1 , Ha-Rim An 2 , Jang-Soon Kwon 1
|
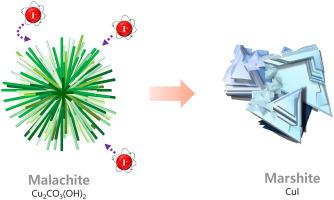
Here we show an innovative way to effectively scavenge highly mobile radioiodide and to dramatically reduce its waste volume through a spontaneous phase transformation. Under an anaerobic condition, as metallic copper (II) was favorably associated with bicarbonate (HCO3−) in solution, a cupriferous carbonate compound (malachite) quickly formed, which was redox-sensitive and transformable to a compact crystal of CuI (marshite). The formation of CuI crystal was principally led by the spontaneous Cu–I redox reaction centering around the copper phase over the presence of sulfate (SO42−). The completely transformed CuI crystal was poorly soluble in water and grew to large microcrystals (∼μm) via a remarkable selectivity for I−. Interestingly, this redox-induced iodide crystallization was rather promoted over the existence of anionic competitors (e.g., HCO3− and SO42−), which usually exist in wastewater and natural water. Unlike the conventional methods, these competing anions positively behaved in our system by supporting that the initial malachite was more apt to be reactive to largely attract highly mobile I−. Under practical environments with various anions, such a selective I− uptake and fixation within a compact crystalline space will be a promising way to effectively remove I− in a great capacity.
中文翻译:

通过低结晶铜相的氧化还原转化使放射性碘化物固定结晶
在这里,我们展示了一种有效清除高流动性放射性碘化物并通过自发相变显着减少其废物量的创新方法。在厌氧条件下,由于金属铜 (II) 与溶液中的碳酸氢盐 (HCO 3 - )有利地结合,快速形成亚铜碳酸盐化合物(孔雀石),该化合物对氧化还原敏感且可转化为致密的 CuI 晶体(marshite) . CuI 晶体的形成主要是由在硫酸盐 (SO 4 2- )存在下以铜相为中心的自发 Cu-I 氧化还原反应引起的。完全转化的 CuI 晶体难溶于水,并通过显着的 I -选择性生长成大的微晶(~μm). 有趣的是,这种氧化还原诱导的碘化物结晶在阴离子竞争者(例如,HCO 3 -和 SO 4 2-)的存在下得到了促进,阴离子竞争者通常存在于废水和天然水中。与传统方法不同,这些竞争阴离子在我们的系统中表现积极,支持初始孔雀石更容易反应以在很大程度上吸引高度移动的 I -。在具有各种阴离子的实际环境中,在紧凑的晶体空间内选择性地吸收和固定I -将是一种有效去除 I -大容量的有前途的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号