Pharmacology & Therapeutics ( IF 13.5 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.pharmthera.2021.107988 Saravana Babu Chidambaram 1 , Musthafa Mohamed Essa 2 , A G Rathipriya 3 , Muhammed Bishir 4 , Bipul Ray 1 , Arehally M Mahalakshmi 4 , A H Tousif 1 , Meena K Sakharkar 5 , Rajpal Singh Kashyap 6 , Robert P Friedland 7 , Tanya M Monaghan 8
|
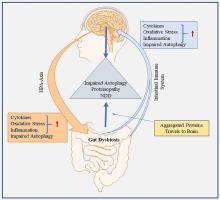
The human microbiota comprises trillions of symbiotic microorganisms and is involved in regulating gastrointestinal (GI), immune, nervous system and metabolic homeostasis. Recent observations suggest a bidirectional communication between the gut microbiota and the brain via immune, circulatory and neural pathways, termed the Gut-Brain Axis (GBA). Alterations in gut microbiota composition, such as seen with an increased number of pathobionts and a decreased number of symbionts, termed gut dysbiosis or microbial intestinal dysbiosis, plays a prominent role in the pathogenesis of central nervous system (CNS)-related disorders. Clinical reports confirm that GI symptoms often precede neurological symptoms several years before the development of neurodegenerative diseases (NDDs). Pathologically, gut dysbiosis disrupts the integrity of the intestinal barrier leading to ingress of pathobionts and toxic metabolites into the systemic circulation causing GBA dysregulation. Subsequently, chronic neuroinflammation via dysregulated immune activation triggers the accumulation of neurotoxic misfolded proteins in and around CNS cells resulting in neuronal death. Emerging evidence links gut dysbiosis to the aggravation and/or spread of proteinopathies from the peripheral nervous system to the CNS and defective autophagy-mediated proteinopathies. This review summarizes the current understanding of the role of gut microbiota in NDDs, and highlights a vicious cycle of gut dysbiosis, immune-mediated chronic neuroinflammation, impaired autophagy and proteinopathies, which contributes to the development of neurodegeneration in Alzheimer's disease, Parkinson's disease, Huntington’s disease, multiple sclerosis, amyotrophic lateral sclerosis and frontotemporal lobar degeneration. We also discuss novel therapeutic strategies targeting the modulation of gut dysbiosis through prebiotics, probiotics, synbiotics or dietary interventions, and faecal microbial transplantation (FMT) in the management of NDDs.
中文翻译:

神经退行性疾病中的肠道生态失调、自噬缺陷和免疫反应改变:恶性循环的故事
人类微生物群由数万亿共生微生物组成,参与调节胃肠道 (GI)、免疫、神经系统和代谢稳态。最近的观察表明,肠道微生物群和大脑之间通过免疫、循环和神经通路进行双向交流,称为肠脑轴 (GBA)。肠道微生物群组成的改变,例如致病菌数量增加和共生菌数量减少,称为肠道菌群失调或微生物肠道菌群失调,在中枢神经系统 (CNS) 相关疾病的发病机制中起着重要作用。临床报告证实,胃肠道症状通常在神经退行性疾病 (NDD) 发展前几年出现在神经系统症状之前。病理上,肠道菌群失调会破坏肠道屏障的完整性,导致致病菌和有毒代谢物进入体循环,导致 GBA 失调。随后,通过失调的免疫激活引起的慢性神经炎症触发了神经毒性错误折叠蛋白在 CNS 细胞内和周围的积累,导致神经元死亡。新出现的证据将肠道菌群失调与蛋白质病从周围神经系统到 CNS 的恶化和/或扩散以及自噬介导的缺陷性蛋白质病联系起来。这篇综述总结了目前对肠道菌群在 NDDs 中作用的认识,并强调了肠道菌群失调、免疫介导的慢性神经炎症、自噬受损和蛋白质病的恶性循环,这有助于阿尔茨海默病神经退行性变的发展,帕金森病、亨廷顿病、多发性硬化、肌萎缩侧索硬化和额颞叶变性。我们还讨论了通过益生元、益生菌、合生元或饮食干预来调节肠道菌群失调的新治疗策略,以及在 NDD 管理中的粪便微生物移植 (FMT)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号