当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New 8-hydroxyquinoline derivatives highlight the potential of this class for treatment of fungal infections
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2021-08-26 , DOI: 10.1039/d0nj06188c Angélica Rocha Joaquim 1, 2 , Paula Reginatto 3 , Marcela Silva Lopes 2 , Luana Candice Genz Bazana 2 , Mariana Pies Gionbelli 1 , Maycon Antonio de Cesare 1, 2 , Taís Fernanda Andrzejewski Kaminski 2 , Mário Lettieri Teixeira 4 , Maxwel Adriano Abegg 5 , Alexandre Meneghello Fuentefria 2, 3 , Saulo Fernandes de Andrade 1, 2, 3
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2021-08-26 , DOI: 10.1039/d0nj06188c Angélica Rocha Joaquim 1, 2 , Paula Reginatto 3 , Marcela Silva Lopes 2 , Luana Candice Genz Bazana 2 , Mariana Pies Gionbelli 1 , Maycon Antonio de Cesare 1, 2 , Taís Fernanda Andrzejewski Kaminski 2 , Mário Lettieri Teixeira 4 , Maxwel Adriano Abegg 5 , Alexandre Meneghello Fuentefria 2, 3 , Saulo Fernandes de Andrade 1, 2, 3
Affiliation
|
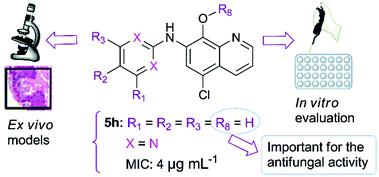
The oral administration of clioquinol – a potent 8-hydroxyquinoline antimicrobial drug – was forbidden due to suspicion of it being the cause of SMON (subacute myelo-optic-neuropathy) in Japan. However, this adverse effect was only observed in Japan, despite the fact that the drug was used worldwide. In addition, studies have shown that vitamin B12 deficiency could be involved in this process. Thus, the interest in the modification of this compound has recently emerged as a strategy to find new drug candidates. In the present work, we have designed and synthesized a novel series of clioquinol derivatives by the modification of the 7-position of this compound. Twenty-one derivatives were prepared from commercially available clioquinol in two or three steps: 8-OH-protection of clioquinol followed by the palladium-catalyzed cross-coupling reaction to introduce the arylamine group at position 7 resulting in derivatives 4a–4j. Finally, deprotection of the hydroxyl group gave derivatives 5a–5i and 10a–10b. The compounds prepared were tested against Candida spp. and dermatophyte isolates by the broth microdilution method. We have found a clear structure–activity relationship. The presence of free hydroxyl in the 8-position and the introduction of the hydrophilic heterocyclic substituent at the 7-position are important for the antifungal activity of this class. Moreover, the introduction of dimethoxy groups at the pyrimidinyl ring attached to the 7-position seems to be promising against dermatophytes and Candida albicans. Among all derivatives, compound 5h presented interesting activity against all fungal species tested (with MIC values of 4 μg mL−1), along with low toxicity in normal cells. This derivative is non-irritant with the absence of topical toxicity. In addition, 5h does not seem to act as an ionophore in fungal cells, similarly to clioquinol. These compounds appear to have a scavenger effect, which suggests their selectivity for fungal cells. Taken together, these findings indicate the potential of 5h to be developed as an antifungal agent.
中文翻译:

新的 8-羟基喹啉衍生物突出了此类治疗真菌感染的潜力
氯碘羟喹(一种强效 8-羟基喹啉抗菌药物)的口服给药被禁止,因为在日本怀疑它是 SMON(亚急性髓视神经病变)的原因。然而,尽管该药物已在世界范围内使用,但这种不良反应仅在日本观察到。此外,研究表明,维生素 B12 缺乏可能与此过程有关。因此,最近对该化合物的修饰产生了兴趣,作为寻找新候选药物的策略。在目前的工作中,我们通过修饰该化合物的 7 位,设计并合成了一系列新的氯碘羟喹衍生物。21 种衍生物是由市售氯碘羟喹通过两步或三步制备的:4a–4j。最后,羟基的脱保护得到衍生物5a-5i和10a-10b。制备的化合物针对念珠菌属进行了测试。通过肉汤微量稀释法分离皮肤癣菌。我们发现了明确的结构-活性关系。8-位游离羟基的存在和7-位亲水性杂环取代基的引入对于此类抗真菌活性很重要。此外,在连接到 7 位的嘧啶环上引入二甲氧基似乎有望对抗皮肤癣菌和白色念珠菌。在所有衍生物中,化合物5h对所有测试的真菌物种表现出有趣的活性(MIC 值为 4 μg mL -1),同时在正常细胞中具有低毒性。该衍生物无刺激性,无局部毒性。此外,与氯碘羟喹类似,5h似乎不会在真菌细胞中充当离子载体。这些化合物似乎具有清除作用,这表明它们对真菌细胞具有选择性。综上所述,这些发现表明5h具有作为抗真菌剂开发的潜力。
更新日期:2021-09-16
中文翻译:

新的 8-羟基喹啉衍生物突出了此类治疗真菌感染的潜力
氯碘羟喹(一种强效 8-羟基喹啉抗菌药物)的口服给药被禁止,因为在日本怀疑它是 SMON(亚急性髓视神经病变)的原因。然而,尽管该药物已在世界范围内使用,但这种不良反应仅在日本观察到。此外,研究表明,维生素 B12 缺乏可能与此过程有关。因此,最近对该化合物的修饰产生了兴趣,作为寻找新候选药物的策略。在目前的工作中,我们通过修饰该化合物的 7 位,设计并合成了一系列新的氯碘羟喹衍生物。21 种衍生物是由市售氯碘羟喹通过两步或三步制备的:4a–4j。最后,羟基的脱保护得到衍生物5a-5i和10a-10b。制备的化合物针对念珠菌属进行了测试。通过肉汤微量稀释法分离皮肤癣菌。我们发现了明确的结构-活性关系。8-位游离羟基的存在和7-位亲水性杂环取代基的引入对于此类抗真菌活性很重要。此外,在连接到 7 位的嘧啶环上引入二甲氧基似乎有望对抗皮肤癣菌和白色念珠菌。在所有衍生物中,化合物5h对所有测试的真菌物种表现出有趣的活性(MIC 值为 4 μg mL -1),同时在正常细胞中具有低毒性。该衍生物无刺激性,无局部毒性。此外,与氯碘羟喹类似,5h似乎不会在真菌细胞中充当离子载体。这些化合物似乎具有清除作用,这表明它们对真菌细胞具有选择性。综上所述,这些发现表明5h具有作为抗真菌剂开发的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号