Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.jmb.2021.167252 Alessandro Strofaldi 1 , Amir R Khan 2 , Jennifer J McManus 3
|
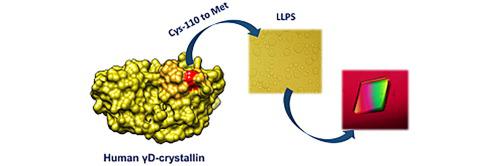
Human γD-crystallin (HGD) has remarkable stability against condensation in the human lens, sometimes over a whole lifetime. The native protein has a surface exposed free cysteine that forms dimers (Benedek, 1997; Ramkumar et al., 1864)1,2 without specific biological function and leads to further protein association and/or aggregation, which creates a paradox for understanding its stability. Previous work has demonstrated that chemical modification of the protein at the free cysteine (C110), increases the temperature at which liquid–liquid phase separation occurs (LLPS), lowers protein solubility and suggests an important role for this amino acid in maintaining its long-term resistance to condensation. Here we demonstrate that mutation of the cysteine does not alter the structure or solubility (liquidus) line for the protein, but dramatically increases the protein crystal nucleation rate following LLPS, suggesting that the free cysteine has a vital role in suppressing crystallization in the human lens.
中文翻译:

表面暴露的游离半胱氨酸抑制人 γD-Crystallin 的结晶
人类 γD-晶状体蛋白 (HGD) 对人晶状体中的冷凝具有显着的稳定性,有时在整个生命周期内。天然蛋白质有一个表面暴露的游离半胱氨酸,形成二聚体 (Benedek, 1997; Ramkumar et al., 1864) 1,2没有特定的生物学功能并导致进一步的蛋白质结合和/或聚集,这为理解其稳定性创造了一个悖论。先前的工作表明,游离半胱氨酸 (C110) 处蛋白质的化学修饰,提高了发生液-液相分离 (LLPS) 的温度,降低了蛋白质的溶解度,并表明该氨基酸在维持其长期长期抗冷凝。在这里,我们证明半胱氨酸的突变不会改变蛋白质的结构或溶解度(液相线)线,但会显着增加 LLPS 后的蛋白质晶体成核率,这表明游离半胱氨酸在抑制人晶状体结晶方面起着至关重要的作用.


























 京公网安备 11010802027423号
京公网安备 11010802027423号