Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.jinorgbio.2021.111604 Jaswir Basran 1 , Elizabeth S Booth 2 , Laura P Campbell 3 , Sarah J Thackray 3 , Mehul H Jesani 4 , Jonathan Clayden 4 , Peter C E Moody 1 , Christopher G Mowat 3 , Hanna Kwon 4 , Emma L Raven 4
|
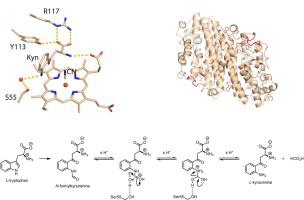
The kynurenine pathway is the major route of tryptophan metabolism. The first step of this pathway is catalysed by one of two heme-dependent dioxygenase enzymes – tryptophan 2,3-dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO) – leading initially to the formation of N-formylkynurenine (NFK). In this paper, we present a crystal structure of a bacterial TDO from X. campestris in complex with l-kynurenine, the hydrolysed product of NFK. l-kynurenine is bound at the active site in a similar location to the substrate (l-Trp). Hydrogen bonding interactions with Arg117 and the heme 7-propionate anchor the l-kynurenine molecule into the pocket. A mechanism for the hydrolysis of NFK in the active site is presented.
中文翻译:

L-犬尿氨酸与 X. campestris 色氨酸 2,3-双加氧酶的结合
犬尿氨酸途径是色氨酸代谢的主要途径。该途径的第一步是由两种血红素依赖性双加氧酶中的一种催化 - 色氨酸 2,3-双加氧酶 (TDO) 和吲哚胺 2,3-双加氧酶 (IDO) - 最初导致N-甲酰基犬尿氨酸 (NFK)的形成. 在本文中,我们展示了来自野菜的细菌 TDO 与NFK 的水解产物l-犬尿氨酸复合的晶体结构。l-犬尿氨酸结合在与底物 ( l - Trp) 相似位置的活性位点。与 Arg117 和血红素 7-丙酸盐的氢键相互作用锚定l-犬尿氨酸分子放入口袋。提出了 NFK 在活性位点水解的机制。

























 京公网安备 11010802027423号
京公网安备 11010802027423号