Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Suppressing Sulfite Dimerization at a Polarized Gold Electrode/Water Solution Interface for High-Quality Gold Electrodeposition
Langmuir ( IF 3.9 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.langmuir.1c01595 Jia-Qiang Yang 1 , Lei Jin 1 , Yuan-Hui Xiao 1 , Huan-Huan Yu 1 , Fang-Zu Yang 1 , Dong-Ping Zhan 1 , De-Yin Wu 1 , Zhong-Qun Tian 1
Langmuir ( IF 3.9 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.langmuir.1c01595 Jia-Qiang Yang 1 , Lei Jin 1 , Yuan-Hui Xiao 1 , Huan-Huan Yu 1 , Fang-Zu Yang 1 , Dong-Ping Zhan 1 , De-Yin Wu 1 , Zhong-Qun Tian 1
Affiliation
|
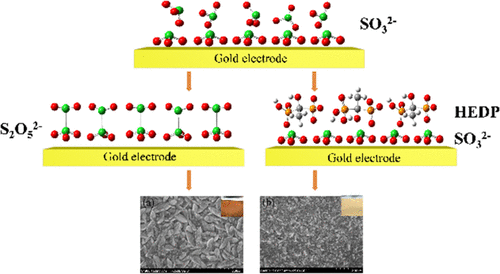
Solid/liquid interfacial structure occupies great importance in chemistry, biology, and materials. In this paper, by combining EC-SERS study and DFT calculation, we reveal the adsorption and dimerization of sulfite (SO32–) at a gold electrode/water solution interface, and establish an adsorption displacement strategy to suppress the dimerization of sulfite. At the gold electrode/sodium sulfite solution interface, at least two layers of SO32– anions are adsorbed on the electrode surface. As the applied potential shifts negatively, the adsorption strength of the first SO32– layer is weakened gradually and then is dimerized with the second orientated SO32– layer to form S2O52–, and S2O52– is further reduced to S2O32–. After hydroxyethylene disphosphonic acid (HEDP) is introduced to the gold electrode/sodium sulfite solution interface, the second oriented SO32– layer is replaced by a HEDP coadsorption layer. This results in the first layer of SO32– being desorbed directly without any structural transformation or chemical reaction as the potential shifts negatively. The suppression of sulfite dimerization by HEDP is more clear at the gold electrode/gold sulfite solution interface owing to the electroreduction of gold ions. Furthermore, the electrochemical studies and electrodeposition experiments show that as the sulfite dimerization reaction is suppressed, the electroreduction of gold ions is accelerated, and the deposited gold coating is bright and dense with finer grains.
中文翻译:

在极化金电极/水溶液界面抑制亚硫酸盐二聚化以实现高质量的金电沉积
固/液界面结构在化学、生物学和材料中占有重要地位。在本文中,通过结合EC-SERS研究和DFT计算,我们揭示了金电极/水溶液界面处亚硫酸盐(SO 3 2-)的吸附和二聚化,并建立了一种吸附置换策略来抑制亚硫酸盐的二聚化。在金电极/亚硫酸钠溶液界面处,电极表面至少吸附了两层 SO 3 2-阴离子。随着外加电位的负移,第一层SO 3 2–的吸附强度逐渐减弱,然后与第二层定向的 SO 3 2–层二聚化形成 S 2O 5 2-和S 2 O 5 2-进一步还原为S 2 O 3 2-。在将羟基乙烯二膦酸 (HEDP) 引入金电极/亚硫酸钠溶液界面后,第二个定向的 SO 3 2-层被 HEDP 共吸附层取代。这导致第一层 SO 3 2–随着电位的负移,无需任何结构转变或化学反应即可直接解吸。由于金离子的电还原,HEDP 对亚硫酸盐二聚化的抑制在金电极/亚硫酸金溶液界面上更为明显。此外,电化学研究和电沉积实验表明,随着亚硫酸盐二聚反应的抑制,金离子的电还原加速,沉积的金涂层光亮致密,晶粒更细。
更新日期:2021-09-28
中文翻译:

在极化金电极/水溶液界面抑制亚硫酸盐二聚化以实现高质量的金电沉积
固/液界面结构在化学、生物学和材料中占有重要地位。在本文中,通过结合EC-SERS研究和DFT计算,我们揭示了金电极/水溶液界面处亚硫酸盐(SO 3 2-)的吸附和二聚化,并建立了一种吸附置换策略来抑制亚硫酸盐的二聚化。在金电极/亚硫酸钠溶液界面处,电极表面至少吸附了两层 SO 3 2-阴离子。随着外加电位的负移,第一层SO 3 2–的吸附强度逐渐减弱,然后与第二层定向的 SO 3 2–层二聚化形成 S 2O 5 2-和S 2 O 5 2-进一步还原为S 2 O 3 2-。在将羟基乙烯二膦酸 (HEDP) 引入金电极/亚硫酸钠溶液界面后,第二个定向的 SO 3 2-层被 HEDP 共吸附层取代。这导致第一层 SO 3 2–随着电位的负移,无需任何结构转变或化学反应即可直接解吸。由于金离子的电还原,HEDP 对亚硫酸盐二聚化的抑制在金电极/亚硫酸金溶液界面上更为明显。此外,电化学研究和电沉积实验表明,随着亚硫酸盐二聚反应的抑制,金离子的电还原加速,沉积的金涂层光亮致密,晶粒更细。


























 京公网安备 11010802027423号
京公网安备 11010802027423号