当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Linear Hydrocarbon Chain Growth from a Molecular Diruthenium Carbide Platform
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c06586 Jun Ohata 1 , Akira Teramoto 1 , Hiroaki Fujita 1 , Shin Takemoto 1 , Hiroyuki Matsuzaka 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c06586 Jun Ohata 1 , Akira Teramoto 1 , Hiroaki Fujita 1 , Shin Takemoto 1 , Hiroyuki Matsuzaka 1
Affiliation
|
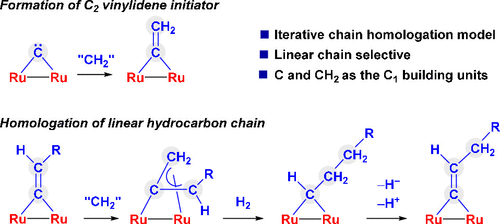
The formation of linear hydrocarbon chains by sequential coupling of C1 units on the metal surface is the central part of the Fischer–Tropsch (F-T) synthesis. Organometallic complexes have provided numerous models of relevant individual C–C coupling events but have failed to reproduce the complete chain lengthening sequence that transforms a linear Cn hydrocarbon chain into its Cn+1 homologue in an iterative fashion. In this work, we demonstrate stepwise growth of linear Cn hydrocarbon chains and their conversion to their Cn+1 homologues via consecutive addition of CH2 units on a molecular diruthenium carbide platform. The chain growth sequence is initiated by the formation of a μ-η1:η1-C═CH2 ligand from a C + CH2 coupling between the μ-carbido complex [(Cp*Ru)2(η-NPh)(μ-C)] (1; Cp* = η5-C5Me5) and Ph2SCH2. Then, the chain propagates via a general C═CHR + CH2 coupling and subsequent hydrogen-assisted isomerization of the resulting allene ligand μ-η1:η3-H2C═C═CHR to a higher vinylidene homologue μ-η1:η1-C═CH(CH2)R. By repeating this reaction sequence, up to C6 chains have been synthesized in a stepwise fashion. The key step of this chain homologation sequence is the selective hydrogenation of the μ-η1:η3-allene unit to the corresponding μ-alkylidene ligand. Isotope labeling and computational studies indicate that this transformation proceeds via the hydrogenation of the allene ligand to a terminal alkene form and its isomerization to the μ-alkylidene ligand facilitated by the coordinatively unsaturated diruthenium platform.
中文翻译:

来自分子碳化二钌平台的线性烃链生长
通过在金属表面上连续偶联 C 1单元形成线性烃链是费托 (FT) 合成的核心部分。有机金属配合物提供了许多相关单个 C-C 偶联事件的模型,但未能重现以迭代方式将线性 C n烃链转化为其 C n +1同系物的完整链延长序列。在这项工作中,我们展示了线性 C n烃链的逐步增长及其通过连续添加 CH 2转化为 C n +1同系物分子碳化二钌平台上的单元。链增长序列由形成了μ-η的发起1:η 1 -C═CH 2从C + CH配体2的μ-碳化复合物之间的耦合[(CP * Ru)的2(η-NPH)( μ-C)] ( 1;Cp* = η 5 -C 5 Me 5 )和Ph 2 SCH 2。然后,链通过一般的 C=CHR + CH 2偶联和随后得到的丙二烯配体 μ-n 1 :η 3 -H 2 C =C=CHR 的氢辅助异构化扩展为更高的亚乙烯基同系物 μ-η 1:η 1 -C=CH(CH 2 )R。通过重复该反应序列,最多可逐步合成C 6链。该链同源序列的关键步骤是将 μ-n 1 :n 3 -丙二烯单元选择性氢化成相应的 μ-亚烷基配体。同位素标记和计算研究表明,这种转化是通过丙二烯配体氢化为末端烯烃形式以及在配位不饱和二钌平台的促进下异构化为 μ-亚烷基配体来进行的。
更新日期:2021-10-06
中文翻译:

来自分子碳化二钌平台的线性烃链生长
通过在金属表面上连续偶联 C 1单元形成线性烃链是费托 (FT) 合成的核心部分。有机金属配合物提供了许多相关单个 C-C 偶联事件的模型,但未能重现以迭代方式将线性 C n烃链转化为其 C n +1同系物的完整链延长序列。在这项工作中,我们展示了线性 C n烃链的逐步增长及其通过连续添加 CH 2转化为 C n +1同系物分子碳化二钌平台上的单元。链增长序列由形成了μ-η的发起1:η 1 -C═CH 2从C + CH配体2的μ-碳化复合物之间的耦合[(CP * Ru)的2(η-NPH)( μ-C)] ( 1;Cp* = η 5 -C 5 Me 5 )和Ph 2 SCH 2。然后,链通过一般的 C=CHR + CH 2偶联和随后得到的丙二烯配体 μ-n 1 :η 3 -H 2 C =C=CHR 的氢辅助异构化扩展为更高的亚乙烯基同系物 μ-η 1:η 1 -C=CH(CH 2 )R。通过重复该反应序列,最多可逐步合成C 6链。该链同源序列的关键步骤是将 μ-n 1 :n 3 -丙二烯单元选择性氢化成相应的 μ-亚烷基配体。同位素标记和计算研究表明,这种转化是通过丙二烯配体氢化为末端烯烃形式以及在配位不饱和二钌平台的促进下异构化为 μ-亚烷基配体来进行的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号