当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How does Lewis acid affect the reactivity of mononuclear high-valent chromium–oxo species? A theoretical study
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-09-15 , DOI: 10.1002/bkcs.12397 Yunhee Choi 1 , Bhawana Pandey 2 , Xiao‐Xi Li 2 , Yong‐Min Lee 2 , Kyung‐Bin Cho 1 , Wonwoo Nam 2
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-09-15 , DOI: 10.1002/bkcs.12397 Yunhee Choi 1 , Bhawana Pandey 2 , Xiao‐Xi Li 2 , Yong‐Min Lee 2 , Kyung‐Bin Cho 1 , Wonwoo Nam 2
Affiliation
|
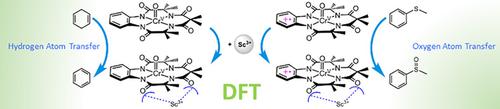
Density functional theory calculations were performed to study the Lewis acid (Sc3+) effects on the reactivity of ultrahigh-valent chromium-oxo species toward both CH bond activation and sulfoxidation reactions. Calculations confirm that the oxidizing power of chromium-oxo species is enhanced by binding Sc3+ ion. In sulfoxidation reactions, especially, binding Sc3+ ion enhances the redox potential of the chromium-oxo species, whereby the activation barrier is decreased dramatically. The details of the reactions obtained by theory are disclosed in this work.
中文翻译:

路易斯酸如何影响单核高价铬氧物种的反应性?理论研究
进行了密度泛函理论计算以研究路易斯酸 (Sc 3+ ) 对超高价铬氧物种对 C H 键活化和磺化氧化反应的反应性的影响。计算证实铬氧物种的氧化能力通过结合 Sc 3+离子而增强。特别是在磺化反应中,结合 Sc 3+离子提高了铬氧物种的氧化还原电位,从而显着降低了活化势垒。在这项工作中公开了通过理论获得的反应的细节。
更新日期:2021-11-22
中文翻译:

路易斯酸如何影响单核高价铬氧物种的反应性?理论研究
进行了密度泛函理论计算以研究路易斯酸 (Sc 3+ ) 对超高价铬氧物种对 C H 键活化和磺化氧化反应的反应性的影响。计算证实铬氧物种的氧化能力通过结合 Sc 3+离子而增强。特别是在磺化反应中,结合 Sc 3+离子提高了铬氧物种的氧化还原电位,从而显着降低了活化势垒。在这项工作中公开了通过理论获得的反应的细节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号