当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determining intrinsic stark tuning rates of adsorbed CO on copper surfaces
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1cy01090e Xiaoxia Chang 1, 2, 3 , Haocheng Xiong 1, 2, 4 , Yifei Xu 1, 2 , Yaran Zhao 3 , Qi Lu 4 , Bingjun Xu 1, 2, 3
Catalysis Science & Technology ( IF 5 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1cy01090e Xiaoxia Chang 1, 2, 3 , Haocheng Xiong 1, 2, 4 , Yifei Xu 1, 2 , Yaran Zhao 3 , Qi Lu 4 , Bingjun Xu 1, 2, 3
Affiliation
|
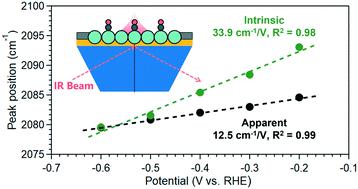
The abrupt change in potential between the electrode and the electrolyte, and the resulting interfacial electric field, is the driving force in electrochemical reactions. For surface mediated electrocatalytic reactions, the interfacial electric field is believed to have a key impact on the stability and reactivity of adsorbed intermediates. However, the exact mechanisms remain a topic of discussion. In this context, reliable measurements of the interfacial electric field are a prerequisite in understanding how it influences the rate and product distribution in electrochemical reactions. The vibrational Stark effect of adsorbates, such as CO, offers an accessible means to assess the interfacial electric field strength by determining the shift of vibrational peaks of the adsorbates with potential, i.e., the Stark tuning rate. However, the vibrational Stark effect could be convoluted with the dynamical dipole coupling effect of the adsorbates on weak binding surfaces such as Cu, thus complicating the determination of the intrinsic Stark tuning rate. In this work, we report a general and effective strategy of determining the intrinsic Stark tuning rate by removing the impact of the dynamical coupling of adsorbed CO on the Cu surface with surface enhanced infrared absorption spectroscopy. A similar intrinsic Stark tuning rate of ∼33 cm V−1 was obtained on oxide-derived Cu in different electrolyte pH of 7.2, 10.9 and 12.9, indicating the pH independence of the interfacial electric field. Investigations on different Cu electrodes show that the intrinsic Stark tuning rates on (electro)chemically deposited films are close to 33 cm V−1, while particulate Cu catalysts show a similar value of ∼68 cm V−1. These observations indicate that aggregate morphology, rather than the size and shape of individual catalyst particles, has a more prominent impact on the interfacial electric field.
中文翻译:

确定铜表面吸附 CO 的固有明显调谐率
电极和电解质之间电位的突然变化以及由此产生的界面电场是电化学反应的驱动力。对于表面介导的电催化反应,界面电场被认为对吸附中间体的稳定性和反应性具有关键影响。然而,确切的机制仍然是讨论的话题。在这种情况下,界面电场的可靠测量是了解它如何影响电化学反应中的速率和产物分布的先决条件。吸附物(如 CO)的振动斯塔克效应提供了一种评估界面电场强度的可行方法,即通过确定吸附物振动峰随电位的位移,即,斯塔克调谐率。然而,振动斯塔克效应可能与吸附物在铜等弱结合表面上的动态偶极耦合效应复杂化,从而使固有斯塔克调谐率的确定变得复杂。在这项工作中,我们报告了一种通用且有效的策略,通过使用表面增强红外吸收光谱去除吸附的 CO 对 Cu 表面的动态耦合的影响来确定内在斯塔克调谐速率。类似的固有斯塔克调谐率约为 33 cm V -1在 7.2、10.9 和 12.9 的不同电解质 pH 值下,在氧化物衍生的 Cu 上获得,表明界面电场的 pH 值独立性。对不同铜电极的研究表明,(电)化学沉积膜的固有斯塔克调谐率接近 33 cm V -1,而颗粒状铜催化剂显示出类似的值,约为 68 cm V -1。这些观察结果表明聚集体形态,而不是单个催化剂颗粒的大小和形状,对界面电场具有更显着的影响。
更新日期:2021-09-15
中文翻译:

确定铜表面吸附 CO 的固有明显调谐率
电极和电解质之间电位的突然变化以及由此产生的界面电场是电化学反应的驱动力。对于表面介导的电催化反应,界面电场被认为对吸附中间体的稳定性和反应性具有关键影响。然而,确切的机制仍然是讨论的话题。在这种情况下,界面电场的可靠测量是了解它如何影响电化学反应中的速率和产物分布的先决条件。吸附物(如 CO)的振动斯塔克效应提供了一种评估界面电场强度的可行方法,即通过确定吸附物振动峰随电位的位移,即,斯塔克调谐率。然而,振动斯塔克效应可能与吸附物在铜等弱结合表面上的动态偶极耦合效应复杂化,从而使固有斯塔克调谐率的确定变得复杂。在这项工作中,我们报告了一种通用且有效的策略,通过使用表面增强红外吸收光谱去除吸附的 CO 对 Cu 表面的动态耦合的影响来确定内在斯塔克调谐速率。类似的固有斯塔克调谐率约为 33 cm V -1在 7.2、10.9 和 12.9 的不同电解质 pH 值下,在氧化物衍生的 Cu 上获得,表明界面电场的 pH 值独立性。对不同铜电极的研究表明,(电)化学沉积膜的固有斯塔克调谐率接近 33 cm V -1,而颗粒状铜催化剂显示出类似的值,约为 68 cm V -1。这些观察结果表明聚集体形态,而不是单个催化剂颗粒的大小和形状,对界面电场具有更显着的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号