Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.jinorgbio.2021.111606 Cheryl L Mathis 1 , Amy M Barrios 1
|
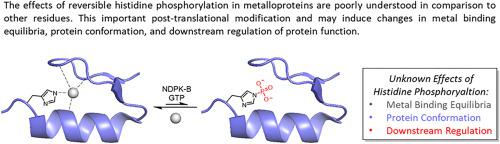
Post-translational modifications (PTMs) are invaluable regulatory tools for the control of catalytic functionality, protein-protein interactions, and signaling pathways. Historically, the study of phosphorylation as a PTM has been focused on serine, threonine, and tyrosine residues. In contrast, the significance of mammalian histidine phosphorylation remains largely unexplored. This gap in knowledge regarding the molecular basis for histidine phosphorylation as a regulatory agent exists in part because of the relative instability of phosphorylated histidine as compared with phosphorylated serine, threonine and tyrosine. However, the unique metal binding abilities of histidine make it one of the most common metal coordinating ligands in nature, and it is interesting to consider how phosphorylation would change the metal coordinating ability of histidine, and consequently, the properties of the phosphorylated metalloprotein. In this review, we examine eleven metalloproteins that have been shown to undergo reversible histidine phosphorylation at or near their metal binding sites. These proteins are described with respect to their biological activity and structure, with a particular emphasis on how phosphohistidine may tune the primary coordination sphere and protein conformation. Furthermore, several common methods, challenges, and limitations of studying sensitive, high affinity metalloproteins are discussed.
中文翻译:

金属蛋白结合位点的组氨酸磷酸化
翻译后修饰 (PTM) 是控制催化功能、蛋白质-蛋白质相互作用和信号通路的宝贵调节工具。从历史上看,磷酸化作为 PTM 的研究一直集中在丝氨酸、苏氨酸和酪氨酸残基上。相比之下,哺乳动物组氨酸磷酸化的意义在很大程度上仍未得到探索。这种关于组氨酸磷酸化作为调节剂的分子基础的知识空白部分是因为与磷酸化丝氨酸、苏氨酸和酪氨酸相比,磷酸化组氨酸的相对不稳定性。然而,组氨酸独特的金属结合能力使其成为自然界中最常见的金属配位体之一,有趣的是,考虑磷酸化如何改变组氨酸的金属配位能力,从而改变磷酸化金属蛋白的性质。在这篇综述中,我们检查了 11 种金属蛋白,这些金属蛋白已被证明在其金属结合位点或其附近发生可逆的组氨酸磷酸化。描述了这些蛋白质的生物活性和结构,特别强调了磷酸组氨酸如何调节初级配位球和蛋白质构象。此外,还讨论了研究敏感、高亲和力金属蛋白的几种常见方法、挑战和局限性。我们检查了 11 种金属蛋白,这些金属蛋白已被证明在其金属结合位点或其附近发生可逆的组氨酸磷酸化。描述了这些蛋白质的生物活性和结构,特别强调了磷酸组氨酸如何调节初级配位球和蛋白质构象。此外,还讨论了研究敏感、高亲和力金属蛋白的几种常见方法、挑战和局限性。我们检查了 11 种金属蛋白,这些金属蛋白已被证明在其金属结合位点或其附近发生可逆的组氨酸磷酸化。描述了这些蛋白质的生物活性和结构,特别强调了磷酸组氨酸如何调节初级配位球和蛋白质构象。此外,还讨论了研究敏感、高亲和力金属蛋白的几种常见方法、挑战和局限性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号