Particuology ( IF 3.5 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.partic.2021.08.009 Han Liu 1 , Xiunan Zhang 1 , Ting Wang 1, 2 , Xin Huang 1, 2 , Kui Chen 1 , Na Wang 1 , Shanshan Yu 1 , Yuyuan Dong 1 , Hongxun Hao 1, 2, 3
|
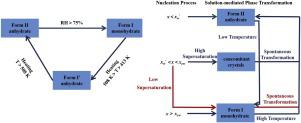
In this work, thermodynamic and kinetic factors that affect formation and phase transformation process of different solid forms of levofloxacin hydrochloride were investigated in detail. Dynamic vapor sorption experiments (DVS) and varying temperature-powder X-ray diffraction (VT-PXRD) experiments were carried out to study moisture-dependent stability, thermal stability as well as the transformation process between Form I and Form II. Critical water activities of levofloxacin hydrochloride were determined in temperature range of 5.0–45.0 °C. Raman spectroscopy was applied to in situ monitor the temperature-induced phase transformation and the hydration process of levofloxacin hydrochloride, and one possible mechanism consisting of multiple chemical equilibria was proposed to analyze the effect of thermodynamic and kinetic factors on the formation and transformation of different solid forms. Results show that the anhydrate, Form II would transform to the monohydrate, Form I more easily with the increasing water content. And the transition point moves toward higher water contents as the temperature increases. The results suggested that, except for thermodynamic factors, kinetic factors also play an essential role in controlling the solid forms of polymorphic compounds.
中文翻译:

盐酸左氧氟沙星相变的热力学和动力学机制
在这项工作中,详细研究了影响盐酸左氧氟沙星不同固体形式的形成和相变过程的热力学和动力学因素。进行动态蒸汽吸附实验 (DVS) 和变温粉末 X 射线衍射 (VT-PXRD) 实验以研究依赖水分的稳定性、热稳定性以及 I 型和 II 型之间的转化过程。盐酸左氧氟沙星的临界水分活度在 5.0–45.0 °C 的温度范围内测定。应用拉曼光谱原位监测盐酸左氧氟沙星的温度诱导相变和水合过程,并提出了一种由多种化学平衡组成的可能机制来分析热力学和动力学因素对不同固体形式的形成和转化的影响。结果表明,随着含水量的增加,无水物II型更容易转化为一水合物I型。随着温度的升高,转变点向更高的水含量移动。结果表明,除了热力学因素外,动力学因素在控制多晶型化合物的固体形式方面也起着重要作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号