当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Are bis(pyridine)iodine(I) complexes applicable for asymmetric halogenation?
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01532j Daniel von der Heiden 1 , Flóra Boróka Németh 2 , Måns Andreasson 3 , Daniel Sethio 1 , Imre Pápai 2, 4 , Mate Erdelyi 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01532j Daniel von der Heiden 1 , Flóra Boróka Németh 2 , Måns Andreasson 3 , Daniel Sethio 1 , Imre Pápai 2, 4 , Mate Erdelyi 1
Affiliation
|
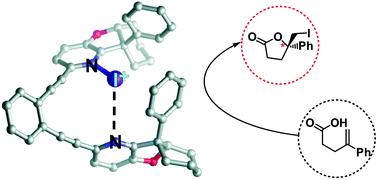
Enantiopure halogenated molecules are of tremendous importance as synthetic intermediates in the construction of pharmaceuticals, fragrances, flavours, natural products, pesticides, and functional materials. Enantioselective halofunctionalizations remain poorly understood and generally applicable procedures are lacking. The applicability of chiral trans-chelating bis(pyridine)iodine(I) complexes in the development of substrate independent, catalytic enantioselective halofunctionalization has been explored herein. Six novel chiral bidentate pyridine donor ligands have been designed, routes for their synthesis developed and their [N–I–N]+-type halogen bond complexes studied by 15N NMR and DFT. The chiral complexes encompassing a halogen bond stabilized iodenium ion are shown to be capable of efficient iodenium transfer to alkenes; however, without enantioselectivity. The lack of stereoselectivity is shown to originate from the availability of multiple ligand conformations of comparable energies and an insufficient steric influence by the chiral ligand. Substrate preorganization by the chiral catalyst appears a necessity for enantioselective halofunctionalization.
中文翻译:

双(吡啶)碘(I)配合物是否适用于不对称卤化?
对映体纯卤化分子作为合成中间体在药物、香料、香精、天然产物、杀虫剂和功能材料的构造中具有极其重要的作用。对映选择性卤代官能化仍然知之甚少,缺乏普遍适用的程序。本文探讨了手性反式螯合双(吡啶)碘( I )配合物在开发与底物无关的催化对映选择性卤代官能化中的适用性。设计了六种新型手性双齿吡啶供体配体,开发了它们的合成路线,并由15研究了它们的 [N-I-N] +型卤素键配合物N核磁共振和密度泛函。包含卤素键稳定的碘鎓离子的手性配合物显示能够有效地将碘鎓转移到烯烃;然而,没有对映选择性。立体选择性的缺乏被证明源于具有可比能量的多个配体构象的可用性和手性配体的空间影响不足。手性催化剂对底物的预组织似乎是对映选择性卤代官能化的必要条件。
更新日期:2021-09-15
中文翻译:

双(吡啶)碘(I)配合物是否适用于不对称卤化?
对映体纯卤化分子作为合成中间体在药物、香料、香精、天然产物、杀虫剂和功能材料的构造中具有极其重要的作用。对映选择性卤代官能化仍然知之甚少,缺乏普遍适用的程序。本文探讨了手性反式螯合双(吡啶)碘( I )配合物在开发与底物无关的催化对映选择性卤代官能化中的适用性。设计了六种新型手性双齿吡啶供体配体,开发了它们的合成路线,并由15研究了它们的 [N-I-N] +型卤素键配合物N核磁共振和密度泛函。包含卤素键稳定的碘鎓离子的手性配合物显示能够有效地将碘鎓转移到烯烃;然而,没有对映选择性。立体选择性的缺乏被证明源于具有可比能量的多个配体构象的可用性和手性配体的空间影响不足。手性催化剂对底物的预组织似乎是对映选择性卤代官能化的必要条件。


























 京公网安备 11010802027423号
京公网安备 11010802027423号