当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mixed Cationic and Anionic Redox in Ni and Co Free Chalcogen-Based Cathode Chemistry for Li-Ion Batteries
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c06828 Sudhan Nagarajan 1 , Sooyeon Hwang 2 , Mahalingam Balasubramanian 3 , Naresh Kumar Thangavel 1 , Leela Mohana Reddy Arava 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/jacs.1c06828 Sudhan Nagarajan 1 , Sooyeon Hwang 2 , Mahalingam Balasubramanian 3 , Naresh Kumar Thangavel 1 , Leela Mohana Reddy Arava 1
Affiliation
|
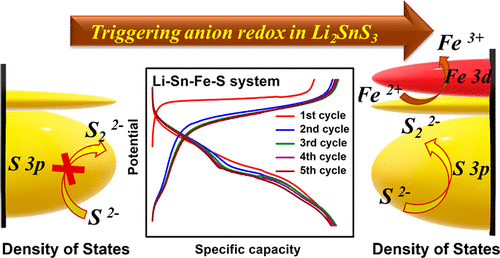
Mixed cationic and anionic redox cathode chemistry is emerging as the conventional cationic redox centers of transition-metal-based layered oxides are reaching their theoretical capacity limit. However, these anionic redox reactions in transition metal oxide-based cathodes attained by taking excess lithium ions have resulted in stability issues due to weak metal–oxygen ligand covalency. Here, we present an alternative approach of improving metal–ligand covalency by introducing a less electronegative chalcogen ligand (sulfur) in the cathode structural framework where the metal d band penetrates into the ligand p band, thereby utilizing reversible mixed anionic and cationic redox chemistry. Through this design strategy, we report the possibility of developing a new family of layered cathode materials when partially filled d orbital redox couples like Fe2+/3+ are introduced in the Li-ion conducting phase (Li2SnS3). Further, the electron energy loss spectroscopy and X-ray absorption near-edge structure analyses are used to qualitatively identify the charge contributors at the metal and ligand sites during Li+ extraction. The detailed high-resolution transmission electron microscopy and high annular dark field-scanning transmission electron microscopy investigations reveal the multi-redox induced structural modifications and its surface amorphization with nanopore formation during cycling. Findings from this study will shed light on designing Ni and Co free chalcogen cathodes and various functional materials in the chalcogen-based dual anionic and cationic redox cathode avenue.
中文翻译:

用于锂离子电池的不含镍和钴的硫属元素基阴极化学中的混合阳离子和阴离子氧化还原
随着过渡金属基层状氧化物的传统阳离子氧化还原中心达到其理论容量极限,混合阳离子和阴离子氧化还原阴极化学正在出现。然而,过渡金属氧化物基正极中的这些阴离子氧化还原反应由于金属-氧配体的共价性较弱,因此通过摄取过量的锂离子而导致稳定性问题。在这里,我们提出了一种通过在阴极结构框架中引入电负性较小的硫属元素配体(硫)来改善金属 - 配体共价的替代方法,其中金属 d 带穿透配体 p 带,从而利用可逆的混合阴离子和阳离子氧化还原化学。通过这种设计策略,2+/3+被引入锂离子导电相(Li 2 SnS 3)。此外,电子能量损失光谱和 X 射线吸收近边缘结构分析用于定性识别 Li +提取过程中金属和配体位点的电荷贡献者。详细的高分辨率透射电子显微镜和高环形暗场扫描透射电子显微镜研究揭示了多氧化还原诱导的结构改性及其在循环过程中纳米孔形成的表面非晶化。这项研究的结果将阐明在基于硫属元素的双阴离子和阳离子氧化还原阴极途径中设计无镍和钴的硫属元素阴极和各种功能材料。
更新日期:2021-09-29
中文翻译:

用于锂离子电池的不含镍和钴的硫属元素基阴极化学中的混合阳离子和阴离子氧化还原
随着过渡金属基层状氧化物的传统阳离子氧化还原中心达到其理论容量极限,混合阳离子和阴离子氧化还原阴极化学正在出现。然而,过渡金属氧化物基正极中的这些阴离子氧化还原反应由于金属-氧配体的共价性较弱,因此通过摄取过量的锂离子而导致稳定性问题。在这里,我们提出了一种通过在阴极结构框架中引入电负性较小的硫属元素配体(硫)来改善金属 - 配体共价的替代方法,其中金属 d 带穿透配体 p 带,从而利用可逆的混合阴离子和阳离子氧化还原化学。通过这种设计策略,2+/3+被引入锂离子导电相(Li 2 SnS 3)。此外,电子能量损失光谱和 X 射线吸收近边缘结构分析用于定性识别 Li +提取过程中金属和配体位点的电荷贡献者。详细的高分辨率透射电子显微镜和高环形暗场扫描透射电子显微镜研究揭示了多氧化还原诱导的结构改性及其在循环过程中纳米孔形成的表面非晶化。这项研究的结果将阐明在基于硫属元素的双阴离子和阳离子氧化还原阴极途径中设计无镍和钴的硫属元素阴极和各种功能材料。

























 京公网安备 11010802027423号
京公网安备 11010802027423号