当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Step-by-step dual stimuli-responsive nanoparticles for efficient bacterial biofilm eradication
Biomaterials Science ( IF 6.6 ) Pub Date : 2021-09-01 , DOI: 10.1039/d1bm01038g Qing Fan 1 , Changrong Wang 1 , Rong Guo 1 , Xinyu Jiang 1 , Wenting Li 1 , Xiangjun Chen 1 , Keke Li 1 , Wei Hong 1
Biomaterials Science ( IF 6.6 ) Pub Date : 2021-09-01 , DOI: 10.1039/d1bm01038g Qing Fan 1 , Changrong Wang 1 , Rong Guo 1 , Xinyu Jiang 1 , Wenting Li 1 , Xiangjun Chen 1 , Keke Li 1 , Wei Hong 1
Affiliation
|
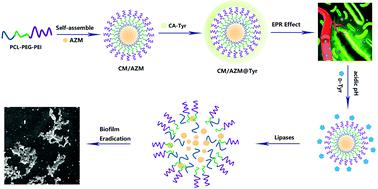
Biofilm-related bacterial infections are extremely resistant to antibiotics, mainly due to the impermeability of the intensive matrices, which allow the bacteria to survive antibiotic treatment. Herein, step-by-step dual stimuli-responsive azithromycin-loaded nanoparticles (CM/AZM@Tyr) was constructed for efficient biofilm eradication. CM/AZM@Tyr was prepared by the self-assembly of poly(ε-caprolactone)-polyethylene glycol-polyethylenimine (PCL-PEG-PEI) into cationic micelles and simultaneously encapsulated AZM into the hydrophobic core, which is further bound with cis-aconityl-D-tyrosine (CA-Tyr) through electrostatic interaction. Upon initial penetration, CM/AZM@Tyr could show step-by-step dual-response to the microenvironment of biofilms. Firstly, the CA-Tyr shell rapidly responded to the acidic microenvironment and released D-Tyr to disassemble the biofilm mass. Then, the exposed cationic CM/AZM micelles could bind firmly to the negatively-charged bacteria cell membrane. With the enzymolysis of the PCL core, the rapidly releasing AZM could kill the bacteria over the depth of biofilms. Massive accumulation was observed in the infected lungs of biofilms-associated lung infection mice after the i.v. injection of CM/Cy5.5@Tyr under the 3D mode of the in vivo Imaging System. Reduced bacterial burden and alleviated fibrosis in the infected lungs were also obtained after treatment with CM/AZM@Tyr mainly due to its intensive penetration in the biofilm and the orderly release of the biofilm dispersant and antimicrobial agents. In summary, this research developed an effective strategy for the treatment of blood-accessible biofilm-induced infections.
中文翻译:

用于有效根除细菌生物膜的分步双刺激响应纳米粒子
生物膜相关的细菌感染对抗生素具有极强的抵抗力,这主要是由于密集基质的不渗透性,这使得细菌能够在抗生素治疗中存活下来。在此,逐步构建了双刺激响应阿奇霉素纳米粒子 (CM/AZM@Tyr) 以有效根除生物膜。CM / AZM @酪氨酸制备由聚自组装(ε -己内酯) -聚乙二醇-聚乙烯亚胺(PCL-PEG-PEI)成阳离子胶束和同时包封AZM到疏水核心,其与进一步结合顺式-乌头-D-酪氨酸(CA-Tyr)通过静电相互作用。初始渗透后,CM/AZM@Tyr 可以对生物膜微环境显示出逐步的双重响应。首先,CA-Tyr 壳快速响应酸性微环境并释放D -Tyr以分解生物膜团。然后,暴露的阳离子 CM/AZM 胶束可以牢固地结合到带负电荷的细菌细胞膜上。随着 PCL 核心的酶解,快速释放的 AZM 可以杀死生物膜深度上的细菌。在体内3D 模式下静脉注射 CM/Cy5.5@Tyr 后,在生物膜相关肺感染小鼠的感染肺中观察到大量积累成像系统。CM/AZM@Tyr 处理后,感染肺部的细菌负荷减少,纤维化减轻,这主要是由于其在生物膜中的强渗透以及生物膜分散剂和抗菌剂的有序释放。总之,这项研究开发了一种有效的策略来治疗血液可及生物膜引起的感染。
更新日期:2021-09-16
中文翻译:

用于有效根除细菌生物膜的分步双刺激响应纳米粒子
生物膜相关的细菌感染对抗生素具有极强的抵抗力,这主要是由于密集基质的不渗透性,这使得细菌能够在抗生素治疗中存活下来。在此,逐步构建了双刺激响应阿奇霉素纳米粒子 (CM/AZM@Tyr) 以有效根除生物膜。CM / AZM @酪氨酸制备由聚自组装(ε -己内酯) -聚乙二醇-聚乙烯亚胺(PCL-PEG-PEI)成阳离子胶束和同时包封AZM到疏水核心,其与进一步结合顺式-乌头-D-酪氨酸(CA-Tyr)通过静电相互作用。初始渗透后,CM/AZM@Tyr 可以对生物膜微环境显示出逐步的双重响应。首先,CA-Tyr 壳快速响应酸性微环境并释放D -Tyr以分解生物膜团。然后,暴露的阳离子 CM/AZM 胶束可以牢固地结合到带负电荷的细菌细胞膜上。随着 PCL 核心的酶解,快速释放的 AZM 可以杀死生物膜深度上的细菌。在体内3D 模式下静脉注射 CM/Cy5.5@Tyr 后,在生物膜相关肺感染小鼠的感染肺中观察到大量积累成像系统。CM/AZM@Tyr 处理后,感染肺部的细菌负荷减少,纤维化减轻,这主要是由于其在生物膜中的强渗透以及生物膜分散剂和抗菌剂的有序释放。总之,这项研究开发了一种有效的策略来治疗血液可及生物膜引起的感染。


























 京公网安备 11010802027423号
京公网安备 11010802027423号