当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facet-Dependent Photoinduced Transformation of Cadmium Sulfide (CdS) Nanoparticles
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.est.1c04026 Meiying Huang 1, 2 , Cun Liu 1 , Peixin Cui 1 , Tongliang Wu 1 , Xionghan Feng 3 , Hui Huang 4 , Jing Zhou 1 , Yujun Wang 1, 2
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.est.1c04026 Meiying Huang 1, 2 , Cun Liu 1 , Peixin Cui 1 , Tongliang Wu 1 , Xionghan Feng 3 , Hui Huang 4 , Jing Zhou 1 , Yujun Wang 1, 2
Affiliation
|
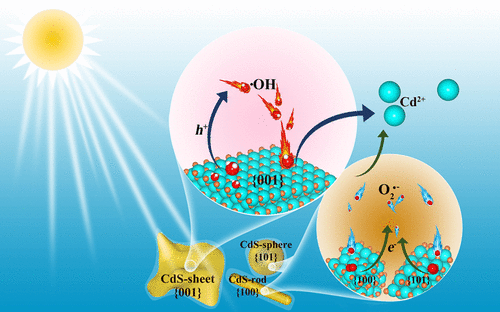
Microbial-mediated transformation of anthropogenic Cd2+ controls its distribution, bioavailability, and potential risks. However, the processes readily form CdS nanoparticles (CdS-NPs), which exhibit dissolution behavior different from that of larger sized particles. Here, we investigated the effects of morphologies and facets of CdS-NPs on their photoinduced dissolution. Three CdS-NPs, CdS-sphere, CdS-rod, and CdS-sheet, and one nanosized biogenic CdS (Bio-CdS) were synthesized with different dominant facets of {101}, {100}, {001}, and {111} and thus distinct surface chemistry. As explored by HRTEM, EPR, and DFT calculations, photogenerated e–/h+ pairs were more likely to generate on CdS-sheet surfaces due to higher surface energies and a narrower band gap, facilitating the formation of •OH and thereby faster dissolution (kobs = 6.126–6.261 × 10–2 h–1). The wider band gaps of CdS-sphere and CdS-rod caused less formation of O2•– and •OH, leading to slower oxidative dissolutions (kobs = 0.090–0.123 and 2.174–3.038 × 10–2 h–1, respectively). Given the similar surface energy as that of CdS-sheet, the dissolution rate of Bio-CdS was close to that of CdS-rod and CdS-sheet, which was 1.6–3.5 times faster than that of larger sized CdS, posing higher environmental risks than thought. Altogether, this work revealed the facet effects on the dissolution of CdS-NPs, manifesting a deeper understanding of metal sulfides’ environmental behaviors.
中文翻译:

硫化镉 (CdS) 纳米粒子的面相关光致转化
人为 Cd 2+ 的微生物介导转化控制其分布、生物利用度和潜在风险。然而,这些过程很容易形成 CdS 纳米颗粒 (CdS-NPs),其表现出与较大尺寸颗粒不同的溶解行为。在这里,我们研究了 CdS-NPs 的形态和面对其光致溶解的影响。合成了三种 CdS-NPs、CdS-sphere、CdS-rod 和 CdS-sheet,以及一种纳米尺寸的生物 CdS(Bio-CdS),具有不同的主要晶面 {101}、{100}、{001} 和 {111 } 从而形成独特的表面化学。根据 HRTEM、EPR 和 DFT 计算,光生 e – /h +由于更高的表面能和更窄的带隙,更容易在 CdS 片表面上生成,从而促进 •OH 的形成,从而更快地溶解(k obs = 6.126–6.261 × 10 –2 h –1)。CdS 球和 CdS 棒的带隙较宽导致 O 2 •–和 •OH 的形成较少,导致氧化溶解较慢(k obs = 0.090–0.123 和 2.174–3.038 × 10 –2 h –1, 分别)。由于表面能与 CdS-sheet 相似,Bio-CdS 的溶解速度接近 CdS-rod 和 CdS-sheet,比更大尺寸的 CdS 快 1.6-3.5 倍,带来更高的环境风险比想。总之,这项工作揭示了对 CdS-NPs 溶解的多方面影响,表明对金属硫化物的环境行为有了更深入的了解。
更新日期:2021-10-06
中文翻译:

硫化镉 (CdS) 纳米粒子的面相关光致转化
人为 Cd 2+ 的微生物介导转化控制其分布、生物利用度和潜在风险。然而,这些过程很容易形成 CdS 纳米颗粒 (CdS-NPs),其表现出与较大尺寸颗粒不同的溶解行为。在这里,我们研究了 CdS-NPs 的形态和面对其光致溶解的影响。合成了三种 CdS-NPs、CdS-sphere、CdS-rod 和 CdS-sheet,以及一种纳米尺寸的生物 CdS(Bio-CdS),具有不同的主要晶面 {101}、{100}、{001} 和 {111 } 从而形成独特的表面化学。根据 HRTEM、EPR 和 DFT 计算,光生 e – /h +由于更高的表面能和更窄的带隙,更容易在 CdS 片表面上生成,从而促进 •OH 的形成,从而更快地溶解(k obs = 6.126–6.261 × 10 –2 h –1)。CdS 球和 CdS 棒的带隙较宽导致 O 2 •–和 •OH 的形成较少,导致氧化溶解较慢(k obs = 0.090–0.123 和 2.174–3.038 × 10 –2 h –1, 分别)。由于表面能与 CdS-sheet 相似,Bio-CdS 的溶解速度接近 CdS-rod 和 CdS-sheet,比更大尺寸的 CdS 快 1.6-3.5 倍,带来更高的环境风险比想。总之,这项工作揭示了对 CdS-NPs 溶解的多方面影响,表明对金属硫化物的环境行为有了更深入的了解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号