当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereoselective Formal [5+2] Cycloaddition of Diazo Arylidene Succinimides-Derived Rhodium Carbenes and Aldehydes: A Route to 2-Benzoxepines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01710 Anna Inyutina 1 , Grigory Kantin 1 , Dmitry Dar In 1 , Mikhail Krasavin 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01710 Anna Inyutina 1 , Grigory Kantin 1 , Dmitry Dar In 1 , Mikhail Krasavin 1
Affiliation
|
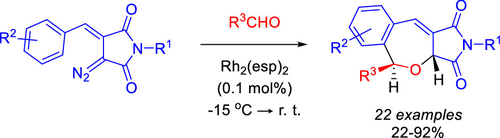
We report on a facile method for the preparation of 2-benzoxepine derivatives as a result of Rh(II)-catalyzed decomposition of diazo arylidene succinimides in the presence of aldehydes. The process is thought to involve the formation of styryl carbonyl ylide which undergoes 1,7-electrocyclization and subsequent 1,5-hydrogen shift. In some cases, the competition of the target reaction and [3+2] dipolar cycloaddition of the intermediate carbonyl ylide to another molecule of diazo substrate was observed. Generally, the desired 2-benzoxepines were isolated in good to high yields and high diastereoselectivity. The developed original approach toward a 2-benzoxepine core via formal [5+2] cycloaddition of styryl carbenoids and aldehydes significantly expands the arsenal of synthetic methods for producing this scaffold.
中文翻译:

重氮亚芳基琥珀酰亚胺衍生的铑卡宾和醛的非对映选择性形式 [5+2] 环加成:2-苯并氧杂环庚烷的途径
我们报告了一种简便的制备 2-苯并氧杂环衍生物的方法,该方法是在醛的存在下,通过 Rh(II) 催化的重氮亚芳基琥珀酰亚胺分解。该过程被认为涉及苯乙烯羰基叶立德的形成,其经历 1,7-电环化和随后的 1,5-氢转移。在某些情况下,观察到目标反应的竞争和中间体羰基叶立德与另一个重氮底物分子的 [3+2] 偶极环加成反应。通常,所需的 2-苯并氧杂环庚烷以良好至高产率和高非对映选择性分离。通过苯乙烯基类卡宾和醛的正式 [5+2] 环加成,开发的 2-苯并氧杂环己酮核心的原始方法显着扩展了用于生产这种支架的合成方法的库。
更新日期:2021-10-01
中文翻译:

重氮亚芳基琥珀酰亚胺衍生的铑卡宾和醛的非对映选择性形式 [5+2] 环加成:2-苯并氧杂环庚烷的途径
我们报告了一种简便的制备 2-苯并氧杂环衍生物的方法,该方法是在醛的存在下,通过 Rh(II) 催化的重氮亚芳基琥珀酰亚胺分解。该过程被认为涉及苯乙烯羰基叶立德的形成,其经历 1,7-电环化和随后的 1,5-氢转移。在某些情况下,观察到目标反应的竞争和中间体羰基叶立德与另一个重氮底物分子的 [3+2] 偶极环加成反应。通常,所需的 2-苯并氧杂环庚烷以良好至高产率和高非对映选择性分离。通过苯乙烯基类卡宾和醛的正式 [5+2] 环加成,开发的 2-苯并氧杂环己酮核心的原始方法显着扩展了用于生产这种支架的合成方法的库。



























 京公网安备 11010802027423号
京公网安备 11010802027423号