当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Consolidating and Managing Data for Drug Development within a Pharmaceutical Laboratory: Comparing the Mapping and Reporting Tools from Software Applications
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.oprd.1c00082 Arvin Moser 1 , Alexander E. Waked 1 , Joseph DiMartino 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.oprd.1c00082 Arvin Moser 1 , Alexander E. Waked 1 , Joseph DiMartino 1
Affiliation
|
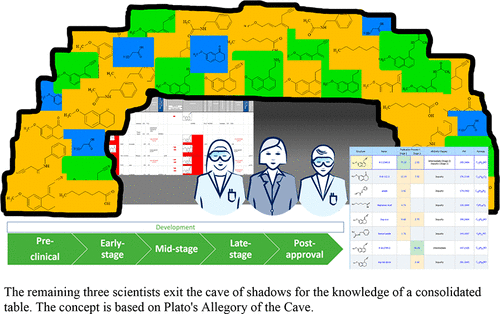
We present a perspective on drug development for the synthesis of an active pharmaceutical ingredient (e.g., agomelatine) within a commercial technology called Luminata and compare the results to the current method of consolidating the reaction data into Microsoft Excel. The Excel document becomes the ultimate repository of information extracted from multiple sources such as the electronic lab notebook, the laboratory information management system, the chromatography data system, in-house databases, and external data. The major needs of a pharmaceutical company are tracking the stages of multiple reactions, calculating the impurity carryover across the stages, and performing structure dereplication for an unknown impurity. As there is no standardized software available to link the different needs throughout the life cycle of process development, there is a demand for mapping tools to consolidate the route for an API synthesis and link it with analytical data while reducing transcription errors and maintaining an audit trail.
中文翻译:

在制药实验室内整合和管理药物开发数据:比较软件应用程序中的绘图和报告工具
我们提出了合成活性药物成分的药物开发的观点(例如, 阿戈美拉汀) 使用一种名为 Luminata 的商业技术,并将结果与当前将反应数据合并到 Microsoft Excel 中的方法进行比较。Excel 文档成为从电子实验室笔记本、实验室信息管理系统、色谱数据系统、内部数据库和外部数据等多个来源提取信息的最终存储库。制药公司的主要需求是跟踪多个反应的阶段,计算跨阶段的杂质残留,以及对未知杂质进行结构去复制。由于没有可用的标准化软件来链接整个过程开发生命周期中的不同需求,
更新日期:2021-10-15
中文翻译:

在制药实验室内整合和管理药物开发数据:比较软件应用程序中的绘图和报告工具
我们提出了合成活性药物成分的药物开发的观点(例如, 阿戈美拉汀) 使用一种名为 Luminata 的商业技术,并将结果与当前将反应数据合并到 Microsoft Excel 中的方法进行比较。Excel 文档成为从电子实验室笔记本、实验室信息管理系统、色谱数据系统、内部数据库和外部数据等多个来源提取信息的最终存储库。制药公司的主要需求是跟踪多个反应的阶段,计算跨阶段的杂质残留,以及对未知杂质进行结构去复制。由于没有可用的标准化软件来链接整个过程开发生命周期中的不同需求,



























 京公网安备 11010802027423号
京公网安备 11010802027423号