Journal of Controlled Release ( IF 10.8 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.jconrel.2021.09.013 Hai Jin 1 , Carole Quesada 2 , Mitra Aliabouzar 2 , Oliver D Kripfgans 3 , Renny T Franceschi 4 , Jianhua Liu 5 , Andrew J Putnam 6 , Mario L Fabiilli 3
|
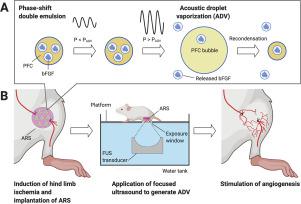
Pro-angiogenic growth factors have been studied as potential therapeutics for cardiovascular diseases like critical limb ischemia (CLI). However, the translation of these factors has remained a challenge, in part, due to problems associated with safe and effective delivery. Here, we describe a hydrogel-based delivery system for growth factors where release is modulated by focused ultrasound (FUS), specifically a mechanism termed acoustic droplet vaporization. With these fibrin-based, acoustically-responsive scaffolds (ARSs), release of a growth factor is non-invasively and spatiotemporally-controlled in an on-demand manner using non-thermal FUS. In vitro studies demonstrated sustained release of basic fibroblast growth factor (bFGF) from the ARSs using repeated applications of FUS. In in vivo studies, ARSs containing bFGF were implanted in mice following induction of hind limb ischemia, a preclinical model of CLI. During the 4-week study, mice in the ARS + FUS group longitudinally exhibited significantly more perfusion and less visible necrosis compared to other experimental groups. Additionally, significantly greater angiogenesis and less fibrosis were observed for the ARS + FUS group. Overall, these results highlight a promising, FUS-based method of delivering a pro-angiogenic growth factor for stimulating angiogenesis and reperfusion in a cardiovascular disease model. More broadly, these results could be used to personalize the delivery of therapeutics in different regenerative applications by actively controlling the release of a growth factor.
中文翻译:

从声响应支架释放碱性成纤维细胞生长因子促进后肢缺血模型中的治疗性血管生成
促血管生成生长因子已被研究作为心血管疾病如严重肢体缺血 (CLI) 的潜在治疗剂。然而,这些因素的转化仍然是一个挑战,部分原因是与安全和有效交付相关的问题。在这里,我们描述了一种基于水凝胶的生长因子递送系统,其中释放由聚焦超声 (FUS) 调制,特别是一种称为声学液滴汽化的机制。使用这些基于纤维蛋白的声学响应支架 (ARS),生长因子的释放是使用非热 FUS 以按需方式无创和时空控制的。体外研究表明,重复应用 FUS 从 ARS 中持续释放碱性成纤维细胞生长因子 (bFGF)。在体内研究中,在诱导后肢缺血(CLI 的临床前模型)后,将含有 bFGF 的 ARS 植入小鼠体内。在为期 4 周的研究中,与其他实验组相比,ARS + FUS 组的小鼠纵向表现出明显更多的灌注和更少的可见坏死。此外,在 ARS + FUS 组中观察到显着更多的血管生成和更少的纤维化。总体而言,这些结果突出了一种有前途的、基于 FUS 的方法,该方法在心血管疾病模型中提供促血管生成生长因子以刺激血管生成和再灌注。更广泛地说,这些结果可用于通过主动控制生长因子的释放来个性化不同再生应用中的治疗方法。与其他实验组相比,ARS + FUS 组的小鼠纵向表现出明显更多的灌注和更少的可见坏死。此外,在 ARS + FUS 组中观察到显着更多的血管生成和更少的纤维化。总体而言,这些结果突出了一种有前途的、基于 FUS 的方法,该方法在心血管疾病模型中提供促血管生成生长因子以刺激血管生成和再灌注。更广泛地说,这些结果可用于通过主动控制生长因子的释放来个性化不同再生应用中的治疗方法。与其他实验组相比,ARS + FUS 组的小鼠纵向表现出明显更多的灌注和更少的可见坏死。此外,在 ARS + FUS 组中观察到显着更多的血管生成和更少的纤维化。总体而言,这些结果突出了一种有前途的、基于 FUS 的方法,该方法在心血管疾病模型中提供促血管生成生长因子以刺激血管生成和再灌注。更广泛地说,这些结果可用于通过主动控制生长因子的释放来个性化不同再生应用中的治疗方法。对于 ARS + FUS 组,观察到显着更多的血管生成和更少的纤维化。总体而言,这些结果突出了一种有前途的、基于 FUS 的方法,该方法在心血管疾病模型中提供促血管生成生长因子以刺激血管生成和再灌注。更广泛地说,这些结果可用于通过主动控制生长因子的释放来个性化不同再生应用中的治疗方法。对于 ARS + FUS 组,观察到显着更多的血管生成和更少的纤维化。总体而言,这些结果突出了一种有前途的、基于 FUS 的方法,该方法在心血管疾病模型中提供促血管生成生长因子以刺激血管生成和再灌注。更广泛地说,这些结果可用于通过主动控制生长因子的释放来个性化不同再生应用中的治疗方法。

























 京公网安备 11010802027423号
京公网安备 11010802027423号