当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Partial Opening of Cytochrome P450cam (CYP101A1) Is Driven by Allostery and Putidaredoxin Binding
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.biochem.1c00406 Simon P Skinner 1 , Alec H Follmer 2 , Marcellus Ubbink 3 , Thomas L Poulos 2, 4, 5 , Jeanine J Houwing-Duistermaat 6 , Emanuele Paci 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-14 , DOI: 10.1021/acs.biochem.1c00406 Simon P Skinner 1 , Alec H Follmer 2 , Marcellus Ubbink 3 , Thomas L Poulos 2, 4, 5 , Jeanine J Houwing-Duistermaat 6 , Emanuele Paci 1
Affiliation
|
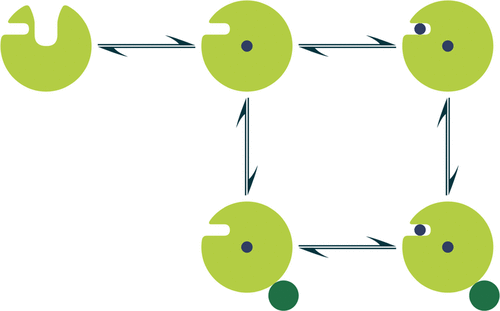
Cytochrome P450cam (CYP101A1) catalyzes the regio- and stereo-specific 5-exo-hydroxylation of camphor via a multistep catalytic cycle that involves two-electron transfer steps, with an absolute requirement that the second electron be donated by the ferrodoxin, putidaredoxin (Pdx). Whether P450cam, once camphor has bound to the active site and the substrate entry channel has closed, opens up upon Pdx binding, during the second electron transfer step, or it remains closed is still a matter of debate. A potential allosteric site for camphor binding has been identified and postulated to play a role in the binding of Pdx. Here, we have revisited paramagnetic NMR spectroscopy data and determined a heterogeneous ensemble of structures that explains the data, provides a complete representation of the P450cam/Pdx complex in solution, and reconciles alternative hypotheses. The allosteric camphor binding site is always present, and the conformational changes induced by camphor binding to this site facilitates Pdx binding. We also determined that the state to which Pdx binds comprises an ensemble of structures that have features of both the open and closed state. These results demonstrate that there is a finely balanced interaction between allosteric camphor binding and the binding of Pdx at high camphor concentrations.
中文翻译:

细胞色素 P450cam (CYP101A1) 的部分开放是由变构和 Putidaredoxin 结合驱动的
细胞色素 P450cam (CYP101A1) 催化区域特异性和立体特异性 5- exo-樟脑的羟基化通过涉及双电子转移步骤的多步催化循环,绝对要求第二个电子由铁氧还蛋白、putidaredoxin (Pdx) 提供。P450cam,一旦樟脑结合到活性位点并且底物进入通道已经关闭,在 Pdx 结合时打开,在第二个电子转移步骤期间,还是保持关闭仍然是一个有争议的问题。樟脑结合的潜在变构位点已被确定并假定在 Pdx 的结合中起作用。在这里,我们重新审视了顺磁 NMR 光谱数据并确定了解释数据的异质结构集合,提供了溶液中 P450cam/Pdx 复合物的完整表示,并调和了替代假设。变构樟脑结合位点始终存在,樟脑与该位点结合引起的构象变化促进了 Pdx 的结合。我们还确定了 Pdx 绑定的状态包含一组具有打开和关闭状态特征的结构。这些结果表明,在高樟脑浓度下,变构樟脑结合与 Pdx 结合之间存在精细平衡的相互作用。
更新日期:2021-10-06
中文翻译:

细胞色素 P450cam (CYP101A1) 的部分开放是由变构和 Putidaredoxin 结合驱动的
细胞色素 P450cam (CYP101A1) 催化区域特异性和立体特异性 5- exo-樟脑的羟基化通过涉及双电子转移步骤的多步催化循环,绝对要求第二个电子由铁氧还蛋白、putidaredoxin (Pdx) 提供。P450cam,一旦樟脑结合到活性位点并且底物进入通道已经关闭,在 Pdx 结合时打开,在第二个电子转移步骤期间,还是保持关闭仍然是一个有争议的问题。樟脑结合的潜在变构位点已被确定并假定在 Pdx 的结合中起作用。在这里,我们重新审视了顺磁 NMR 光谱数据并确定了解释数据的异质结构集合,提供了溶液中 P450cam/Pdx 复合物的完整表示,并调和了替代假设。变构樟脑结合位点始终存在,樟脑与该位点结合引起的构象变化促进了 Pdx 的结合。我们还确定了 Pdx 绑定的状态包含一组具有打开和关闭状态特征的结构。这些结果表明,在高樟脑浓度下,变构樟脑结合与 Pdx 结合之间存在精细平衡的相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号