当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical trigger-enabled bioconjugation reaction
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1ob01177d Fayang Xie 1 , Xiangqian Jia 1 , Zhu Zhu 1 , Yunfei Wu 1 , Haolin Jiang 1, 2 , Hongzhi Yang 1 , Yu Cao 3 , Rui Zhu 1, 4 , Bing Zhou 3 , Juanjuan Du 1 , Yefeng Tang 1
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2021-09-02 , DOI: 10.1039/d1ob01177d Fayang Xie 1 , Xiangqian Jia 1 , Zhu Zhu 1 , Yunfei Wu 1 , Haolin Jiang 1, 2 , Hongzhi Yang 1 , Yu Cao 3 , Rui Zhu 1, 4 , Bing Zhou 3 , Juanjuan Du 1 , Yefeng Tang 1
Affiliation
|
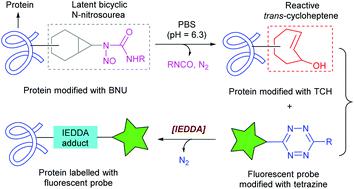
Development of conceptually novel and practically useful bioconjugation reactions has been an intense pursuit of chemical biology research. Herein, we report an unprecedented bioconjugation reaction that hinges on a chemical trigger-enabled inverse-electron-demand Diels–Alder (IEDDA) cycloaddition of trans-cycloheptene (TCH) with tetrazine. Unlike the conventional strain-promoted bioconjugation reactions that utilize built-in strained alkenes as reactants, the current one features a “trigger-release-conjugate” reaction model, in which a highly strained TCH species is released from a bench-stable bicyclic N-nitrosourea (BNU) derivative upon treatment with an external stimulus. It is noteworthy that the reactivity–stability balance of BNU derivatives could be tuned by manipulating their N-1 substituents. As a proof-of-concept case, this new chemical trigger-enabled IEDDA reaction has been applied to in vitro protein labeling and pretargeted cell imaging. This work opens a new avenue to utilize BNU derivatives as a new class of chemical reporters in bioconjugate chemistry.
中文翻译:

化学触发启用的生物共轭反应
开发概念新颖且实用的生物共轭反应一直是化学生物学研究的强烈追求。在这里,我们报告了一种前所未有的生物共轭反应,该反应取决于反式-环庚烯(TCH) 与四嗪的化学触发反电子需求 Diels-Alder (IEDDA) 环加成。与利用内置应变烯烃作为反应物的传统应变促进生物共轭反应不同,目前的反应模型具有“触发-释放-共轭”反应模型,其中高度应变的 TCH 物种从稳定的双环N中释放出来。-亚硝基脲 (BNU) 衍生物在用外部刺激治疗后。值得注意的是,可以通过操纵其 N-1 取代基来调整 BNU 衍生物的反应性-稳定性平衡。作为概念验证案例,这种新的化学触发 IEDDA 反应已应用于体外蛋白质标记和预靶向细胞成像。这项工作为利用 BNU 衍生物作为生物共轭化学中的一类新化学报告分子开辟了一条新途径。
更新日期:2021-09-14
中文翻译:

化学触发启用的生物共轭反应
开发概念新颖且实用的生物共轭反应一直是化学生物学研究的强烈追求。在这里,我们报告了一种前所未有的生物共轭反应,该反应取决于反式-环庚烯(TCH) 与四嗪的化学触发反电子需求 Diels-Alder (IEDDA) 环加成。与利用内置应变烯烃作为反应物的传统应变促进生物共轭反应不同,目前的反应模型具有“触发-释放-共轭”反应模型,其中高度应变的 TCH 物种从稳定的双环N中释放出来。-亚硝基脲 (BNU) 衍生物在用外部刺激治疗后。值得注意的是,可以通过操纵其 N-1 取代基来调整 BNU 衍生物的反应性-稳定性平衡。作为概念验证案例,这种新的化学触发 IEDDA 反应已应用于体外蛋白质标记和预靶向细胞成像。这项工作为利用 BNU 衍生物作为生物共轭化学中的一类新化学报告分子开辟了一条新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号