当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One Week Sustained In Vivo Therapeutic Release and Safety of Novel Extended-Wear Silicone Hydrogel Contact Lenses
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-14 , DOI: 10.1002/adhm.202101263 Stephen A DiPasquale 1, 2 , Liana D Wuchte 2 , Robert J Mosley 2 , Renee M Demarest 3 , Meredith L Voyles 4 , Mark E Byrne 1, 2, 5
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-14 , DOI: 10.1002/adhm.202101263 Stephen A DiPasquale 1, 2 , Liana D Wuchte 2 , Robert J Mosley 2 , Renee M Demarest 3 , Meredith L Voyles 4 , Mark E Byrne 1, 2, 5
Affiliation
|
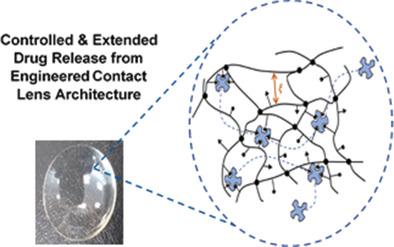
Since the seminal work of Wichterle in 1965 describing the first soft contact lenses and their potential for ocular drug delivery, the field has yet to realize his vision. Maintaining all lens commercial properties combined with a mechanism for controlled drug release of therapeutically relevant concentrations for duration of wear is a major challenge. Here, successful in vivo week-long sustained release of a small molecular weight therapeutic in rabbits from extended-wear silicone hydrogel contact lenses meeting all commercial specifications by utilizing a novel macromolecular memory strategy is reported for the first time. Lens-treated eyes show a continuous, therapeutically relevant bromfenac tear concentration of 256.4 ± 23.1 µg mL−1 for 8 days. Bromday (bromfenac ophthalmic solution, 0.09%, Bausch+Lomb) topical drops exhibit a quick peak concentration of 269.3 ± 85.7 µg mL−1 and 100 min duration. Bioavailability (AUC0-8days) and mean residence time of lenses are 26 and 155 times higher than drops, respectively. Lenses are safe, well tolerated, and no corneal histological differences are observed. This work highlights the enormous potential of drug releasing lenses as a platform strategy, and offers a new dropless clinical strategy for post-cataract, uveitis, post-LASIK, and corneal abrasion treatment.
中文翻译:

新型长效硅水凝胶隐形眼镜的体内持续治疗释放和安全性一周
自 1965 年 Wichterle 的开创性工作描述了第一款软性隐形眼镜及其用于眼部药物输送的潜力以来,该领域尚未实现他的愿景。保持所有镜片的商业特性以及在佩戴期间控制药物释放治疗相关浓度的机制是一项重大挑战。在这里,首次报道了通过利用一种新的大分子记忆策略,从符合所有商业规格的长戴型硅水凝胶隐形眼镜中成功地在兔子体内持续释放一种小分子量治疗剂。经镜片处理的眼睛显示出持续的、治疗相关的溴芬酸泪液浓度为 256.4 ± 23.1 µg mL -18天。Bromday(溴芬酸滴眼液,0.09%,Bausch+Lomb)局部滴剂的快速峰值浓度为 269.3 ± 85.7 µg mL -1,持续时间为 100 分钟。镜片的生物利用度(AUC 0-8 天)和平均停留时间分别是滴剂的 26 倍和 155 倍。镜片是安全的,耐受性良好,并且没有观察到角膜组织学差异。这项工作突出了药物释放镜片作为平台策略的巨大潜力,并为白内障术后、葡萄膜炎、LASIK 术后和角膜擦伤治疗提供了一种新的无滴临床策略。
更新日期:2021-09-14
中文翻译:

新型长效硅水凝胶隐形眼镜的体内持续治疗释放和安全性一周
自 1965 年 Wichterle 的开创性工作描述了第一款软性隐形眼镜及其用于眼部药物输送的潜力以来,该领域尚未实现他的愿景。保持所有镜片的商业特性以及在佩戴期间控制药物释放治疗相关浓度的机制是一项重大挑战。在这里,首次报道了通过利用一种新的大分子记忆策略,从符合所有商业规格的长戴型硅水凝胶隐形眼镜中成功地在兔子体内持续释放一种小分子量治疗剂。经镜片处理的眼睛显示出持续的、治疗相关的溴芬酸泪液浓度为 256.4 ± 23.1 µg mL -18天。Bromday(溴芬酸滴眼液,0.09%,Bausch+Lomb)局部滴剂的快速峰值浓度为 269.3 ± 85.7 µg mL -1,持续时间为 100 分钟。镜片的生物利用度(AUC 0-8 天)和平均停留时间分别是滴剂的 26 倍和 155 倍。镜片是安全的,耐受性良好,并且没有观察到角膜组织学差异。这项工作突出了药物释放镜片作为平台策略的巨大潜力,并为白内障术后、葡萄膜炎、LASIK 术后和角膜擦伤治疗提供了一种新的无滴临床策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号