当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of Mutualism in Organic Synthetic Chemistry: Mutually Promoted C−H Functionalization of Indole and Reduction of Quinoline
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-14 , DOI: 10.1002/adsc.202100819 Sutao Zhang 1 , Hai Xu 1 , Jianghua He 1 , Yuetao Zhang 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-09-14 , DOI: 10.1002/adsc.202100819 Sutao Zhang 1 , Hai Xu 1 , Jianghua He 1 , Yuetao Zhang 2
Affiliation
|
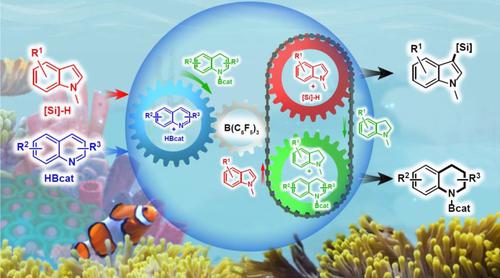
Here we reported a one-pot, metal-free B(C6F5)3-catalyzed strategy for simultaneous synthesis of C3-regioselective functionalization of indoles and complete reduction of quinolines. It turned out that by sharing a quinolinium hydridoborate intermediate, the original determining steps with high energy barrier in both the convergent disproportionation of indole and reduction of quinoline could be realized at room temperature, thus furnishing both the C3-borylated (or silylated) indoles and N-borylated tetrahydroquinolines in up to 98% yields at room temperature. Mechanistic studies suggested that both reactions would consume a product generated from the other reaction such that they can mutually promote each other, thus producing desirable products in a high atom-economy and low energy-cost manner. This strategy opened the gate to introducing mutualism to the field of chemistry.
中文翻译:

互利共生在有机合成化学中的应用:相互促进吲哚的C-H官能化和喹啉的还原
在这里,我们报道了一种单锅、无金属 B(C 6 F 5 ) 3催化策略,用于同时合成吲哚的 C3 区域选择性功能化和喹啉的完全还原。结果表明,通过共享氢硼酸喹啉中间体,可以在室温下实现吲哚的收敛歧化和喹啉的还原中具有高能垒的原始决定步骤,从而提供C3-硼化(或甲硅烷基化)吲哚和N-硼化四氢喹啉在室温下的产率高达 98%。机理研究表明,这两个反应都会消耗另一个反应产生的产物,这样它们就可以相互促进,从而以高原子经济性和低能源成本的方式生产出理想的产物。这一策略打开了将互惠主义引入化学领域的大门。
更新日期:2021-09-14
中文翻译:

互利共生在有机合成化学中的应用:相互促进吲哚的C-H官能化和喹啉的还原
在这里,我们报道了一种单锅、无金属 B(C 6 F 5 ) 3催化策略,用于同时合成吲哚的 C3 区域选择性功能化和喹啉的完全还原。结果表明,通过共享氢硼酸喹啉中间体,可以在室温下实现吲哚的收敛歧化和喹啉的还原中具有高能垒的原始决定步骤,从而提供C3-硼化(或甲硅烷基化)吲哚和N-硼化四氢喹啉在室温下的产率高达 98%。机理研究表明,这两个反应都会消耗另一个反应产生的产物,这样它们就可以相互促进,从而以高原子经济性和低能源成本的方式生产出理想的产物。这一策略打开了将互惠主义引入化学领域的大门。



























 京公网安备 11010802027423号
京公网安备 11010802027423号