当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Cu(II) and Zn(II) on PbO2 Reductive Dissolution under Drinking Water Conditions: Short-term Inhibition and Long-term Enhancement
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.est.1c04887 Weiyi Pan 1 , Greg J Ledingham 2 , Jeffrey G Catalano 2 , Daniel E Giammar 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.est.1c04887 Weiyi Pan 1 , Greg J Ledingham 2 , Jeffrey G Catalano 2 , Daniel E Giammar 1
Affiliation
|
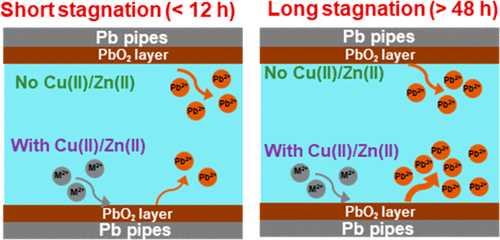
Lead oxide (PbO2) has the lowest solubility with free chlorine among Pb corrosion products, but depletion of free chlorine or a switch from free chlorine to monochloramine can cause its reductive dissolution. We previously reported that Cu(II) and Zn(II) inhibited PbO2 reductive dissolution within 12 h. Here, we expanded on this work by performing longer duration experiments and further exploring the underlying mechanisms. Between 12 and 48 h, Cu(II) and Zn(II) had no discernible effect on PbO2 reductive dissolution. From 48 to 192 h, Cu(II) and Zn(II) enhanced PbO2 reductive dissolution. Dissolved oxygen (DO) concentrations followed the same trends as PbO2 reductive dissolution, indicating that the DO was produced by PbO2 reductive dissolution. On the basis of extended X-ray absorption fine structure spectra, we hypothesize that the inhibitory effect of Cu(II) and Zn(II) on PbO2 reductive dissolution (<12 h) is caused by decreasing abundance of protonated sites on the PbO2 surface. The enhanced dissolution (>48 h) may be caused by competitive adsorption of Cu(II) and Zn(II) with Pb(II), which could limit the adsorption of Pb(II) onto PbO2 that could otherwise inhibit reductive dissolution. This study indicates that stagnation time plays a vital role in determining cations’ effects on the stability of Pb corrosion products.
中文翻译:

Cu(II)和Zn(II)对饮用水条件下PbO2还原溶解的影响:短期抑制和长期增强
在 Pb 腐蚀产物中,氧化铅 (PbO 2 ) 与游离氯的溶解度最低,但游离氯的消耗或从游离氯转换为一氯胺会导致其还原溶解。我们之前报道过,Cu(II) 和 Zn(II)在 12 小时内抑制 PbO 2还原溶解。在这里,我们通过进行更长时间的实验并进一步探索潜在机制来扩展这项工作。在 12 和 48 小时之间,Cu(II) 和 Zn(II) 对 PbO 2还原溶解没有明显影响。从 48 到 192 小时,Cu(II) 和 Zn(II) 增强了 PbO 2还原溶解。溶解氧 (DO) 浓度遵循与 PbO 2相同的趋势还原溶解,表明溶解氧是由 PbO 2还原溶解产生的。基于扩展的 X 射线吸收精细结构光谱,我们假设 Cu(II) 和 Zn(II) 对 PbO 2还原溶解(<12 h)的抑制作用是由于 PbO 上质子化位点丰度的降低引起的2表面。溶解增强(>48 小时)可能是由于 Cu(II) 和 Zn(II) 与 Pb(II) 的竞争吸附引起的,这可能会限制 Pb(II) 吸附到 PbO 2 上,否则会抑制还原溶解。该研究表明,停滞时间在确定阳离子对 Pb 腐蚀产物稳定性的影响方面起着至关重要的作用。
更新日期:2021-11-02
中文翻译:

Cu(II)和Zn(II)对饮用水条件下PbO2还原溶解的影响:短期抑制和长期增强
在 Pb 腐蚀产物中,氧化铅 (PbO 2 ) 与游离氯的溶解度最低,但游离氯的消耗或从游离氯转换为一氯胺会导致其还原溶解。我们之前报道过,Cu(II) 和 Zn(II)在 12 小时内抑制 PbO 2还原溶解。在这里,我们通过进行更长时间的实验并进一步探索潜在机制来扩展这项工作。在 12 和 48 小时之间,Cu(II) 和 Zn(II) 对 PbO 2还原溶解没有明显影响。从 48 到 192 小时,Cu(II) 和 Zn(II) 增强了 PbO 2还原溶解。溶解氧 (DO) 浓度遵循与 PbO 2相同的趋势还原溶解,表明溶解氧是由 PbO 2还原溶解产生的。基于扩展的 X 射线吸收精细结构光谱,我们假设 Cu(II) 和 Zn(II) 对 PbO 2还原溶解(<12 h)的抑制作用是由于 PbO 上质子化位点丰度的降低引起的2表面。溶解增强(>48 小时)可能是由于 Cu(II) 和 Zn(II) 与 Pb(II) 的竞争吸附引起的,这可能会限制 Pb(II) 吸附到 PbO 2 上,否则会抑制还原溶解。该研究表明,停滞时间在确定阳离子对 Pb 腐蚀产物稳定性的影响方面起着至关重要的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号