当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Aqueous Mg2+-Based Dual-Ion Battery with High Power Density
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2021-09-13 , DOI: 10.1002/adfm.202107523 Yunpei Zhu 1 , Jun Yin 2 , Abdul‐Hamid Emwas 3 , Omar F. Mohammed 2 , Husam N. Alshareef 1
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2021-09-13 , DOI: 10.1002/adfm.202107523 Yunpei Zhu 1 , Jun Yin 2 , Abdul‐Hamid Emwas 3 , Omar F. Mohammed 2 , Husam N. Alshareef 1
Affiliation
|
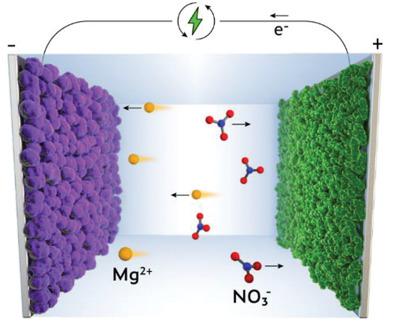
Rechargeable Mg batteries promise low-cost, safe, and high-energy alternatives to Li-ion batteries. However, the high polarization strength of Mg2+ leads to its strong interaction with electrode materials and electrolyte molecules, resulting in sluggish Mg2+ dissociation and diffusion as well as insufficient power density and cycling stability. Here an aqueous Mg2+-based dual-ion battery is reported to bypass the penalties of slow dissociation and solid-state diffusion. This battery chemistry utilizes fast redox reactions on the polymer electrodes, i.e., anion (de)doping on the polyaniline (PANI) cathode and (de)enolization upon incorporating Mg2+ on the polyimide anode. The kinetically favored and stable electrodes depend on designing a saturated aqueous electrolyte of 4.5 m Mg(NO3)2. The concentrated electrolyte suppresses the irreversible deprotonation reaction of the PANI cathode to enable excellent stability (a lifespan of over 10 000 cycles) and rate performance (33% capacity retention at 500 C) and avoids the anodic parasitic reaction of nitrate reduction to deliver the stable polyimide anode (86.2% capacity retention after 6000 cycles). The resultant full Mg2+-based dual-ion battery shows a high specific power of 10 826 W kg−1, competitive with electrochemical supercapacitors. The electrolyte and electrode chemistries elucidated in this study provide an alternative approach to developing better-performing Mg-based batteries.
中文翻译:

一种高功率密度的基于Mg2+的水基双离子电池
可充电镁电池有望成为锂离子电池的低成本、安全和高能量替代品。然而,Mg 2+的高极化强度导致其与电极材料和电解质分子的强烈相互作用,导致Mg 2+解离和扩散缓慢以及功率密度和循环稳定性不足。据报道,一种基于Mg 2+的水性双离子电池绕过了缓慢解离和固态扩散的不利影响。这种电池化学利用聚合物电极上的快速氧化还原反应,即在聚苯胺 (PANI) 阴极上进行阴离子(脱)掺杂和在掺入 Mg 2+后进行(脱)烯醇化在聚酰亚胺阳极上。动力学有利且稳定的电极取决于设计 4.5 m Mg(NO 3 ) 2的饱和含水电解质。浓缩电解质抑制了 PANI 正极的不可逆去质子化反应,以实现出色的稳定性(寿命超过 10 000 次循环)和倍率性能(500 C 下容量保持率为 33%),并避免硝酸盐还原的阳极寄生反应,以提供稳定的聚酰亚胺阳极(6000 次循环后容量保持率为 86.2%)。由此产生的全 Mg 2+基双离子电池显示出 10 826 W kg -1的高比功率,与电化学超级电容器竞争。本研究中阐明的电解质和电极化学物质为开发性能更好的镁基电池提供了一种替代方法。
更新日期:2021-09-13
中文翻译:

一种高功率密度的基于Mg2+的水基双离子电池
可充电镁电池有望成为锂离子电池的低成本、安全和高能量替代品。然而,Mg 2+的高极化强度导致其与电极材料和电解质分子的强烈相互作用,导致Mg 2+解离和扩散缓慢以及功率密度和循环稳定性不足。据报道,一种基于Mg 2+的水性双离子电池绕过了缓慢解离和固态扩散的不利影响。这种电池化学利用聚合物电极上的快速氧化还原反应,即在聚苯胺 (PANI) 阴极上进行阴离子(脱)掺杂和在掺入 Mg 2+后进行(脱)烯醇化在聚酰亚胺阳极上。动力学有利且稳定的电极取决于设计 4.5 m Mg(NO 3 ) 2的饱和含水电解质。浓缩电解质抑制了 PANI 正极的不可逆去质子化反应,以实现出色的稳定性(寿命超过 10 000 次循环)和倍率性能(500 C 下容量保持率为 33%),并避免硝酸盐还原的阳极寄生反应,以提供稳定的聚酰亚胺阳极(6000 次循环后容量保持率为 86.2%)。由此产生的全 Mg 2+基双离子电池显示出 10 826 W kg -1的高比功率,与电化学超级电容器竞争。本研究中阐明的电解质和电极化学物质为开发性能更好的镁基电池提供了一种替代方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号