Renewable Energy ( IF 8.7 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.renene.2021.08.098 Quoc Khanh Tran 1 , Thuan Anh Vo 1 , Hoang Vu Ly 1, 2 , Byeongwan Kwon 1 , Kwang Ho Kim 3 , Seung-Soo Kim 1 , Jinsoo Kim 2
|
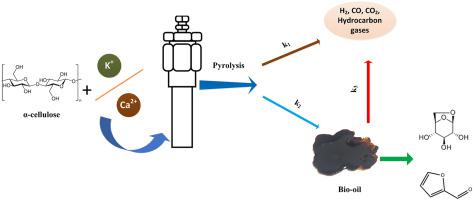
Cellulose accounts for the largest proportion of lignocellulosic biomass. Herein, experimental and simulation studies are used to deeply understand the kinetic characteristics of the thermal decomposition of α-cellulose. The simulated data is in good agreement with the experimental data in the aspects of the conversion and the conversion rate versus temperature. The decomposition of α-cellulose, mainly occurring at 270–420 °C, induced an apparent activation energy ranging from 175.42 kJ/mol to 197.73 kJ/mol at a conversion of 10–90%. With 0.1–0.2 wt% K or Ca impregnation into the α-cellulose, the mean activation energy for pyrolysis was lowered (from 181.47 kJ/mol (for α-cellulose) to 141.11 kJ/mol (for 0.2 wt% K/α-cellulose) and 159.46 kJ/mol (for 0.1 wt% Ca/α-cellulose)) and higher amounts of liquid and gas products were produced. Furthermore, the addition of potassium and calcium increased the production of lower molecular weight components, such as furfural and its derivatives. The kinetic parameters of the α-cellulose pyrolysis were determined based on a nonlinear least-squares regression of the experimental data assuming first-order kinetics and correlated with the simulated result. The kinetic rate constants indicate that the predominant reaction pathway is from α-cellulose into a liquid product, rather than from α-cellulose into a gas product.
中文翻译:

α-纤维素的热解动力学和产物分布:钾和钙浸渍的影响
纤维素占木质纤维素生物质的最大比例。在此,通过实验和模拟研究深入了解α-纤维素热分解的动力学特性。模拟数据在转化率和转化率与温度的关系方面与实验数据非常吻合。α-纤维素的分解,主要发生在 270–420 °C,在 10–90% 的转化率下诱导表观活化能范围为 175.42 kJ/mol 到 197.73 kJ/mol。随着 0.1–0.2 wt% K 或 Ca 浸渍到 α-纤维素中,热解的平均活化能降低(从 181.47 kJ/mol(对于 α-纤维素)到 141.11 kJ/mol(对于 0.2 wt% K/α-纤维素)和 159.46 kJ/mol(对于 0.1 wt% Ca/α-纤维素)和更多的液体和气体产品。此外,钾和钙的添加增加了低分子量组分的产生,例如糠醛及其衍生物。α-纤维素热解的动力学参数是基于假设一级动力学的实验数据的非线性最小二乘回归确定的,并与模拟结果相关。动力学速率常数表明主要的反应途径是从 α-纤维素转化为液体产品,而不是从 α-纤维素转化为气体产品。α-纤维素热解的动力学参数是基于假设一级动力学的实验数据的非线性最小二乘回归确定的,并与模拟结果相关。动力学速率常数表明主要的反应途径是从 α-纤维素转化为液体产品,而不是从 α-纤维素转化为气体产品。α-纤维素热解的动力学参数是基于假设一级动力学的实验数据的非线性最小二乘回归确定的,并与模拟结果相关。动力学速率常数表明主要的反应途径是从 α-纤维素转化为液体产品,而不是从 α-纤维素转化为气体产品。



























 京公网安备 11010802027423号
京公网安备 11010802027423号