当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A photo and tumor microenvironment activated nano-enzyme with enhanced ROS generation and hypoxia relief for efficient cancer therapy
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2021-08-27 , DOI: 10.1039/d1tb01437d Yali Chen 1 , Yujun Cai 1 , Xingsu Yu 2 , Hong Xiao 1 , Haozhe He 1 , Zecong Xiao 1, 3 , Yong Wang 4 , Xintao Shuai 1
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2021-08-27 , DOI: 10.1039/d1tb01437d Yali Chen 1 , Yujun Cai 1 , Xingsu Yu 2 , Hong Xiao 1 , Haozhe He 1 , Zecong Xiao 1, 3 , Yong Wang 4 , Xintao Shuai 1
Affiliation
|
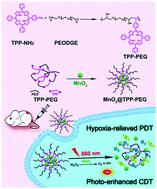
Reactive oxygen species (ROS) mediated tumor therapy strategies have exhibited great prospects and attracted increasing attention, among which photodynamic therapy (PDT) has been well-established. However, the anticancer effects of PDT are greatly limited by the hypoxic tumor microenvironment (TME). Hence, exploring a therapeutic strategy that can relieve tumor hypoxia is regarded as the key to overcoming this problem. Herein, we develop a novel nano-enzyme (MnO2@TPP-PEG) that can accurately conduct tumor-specific catalysis of H2O2 to produce oxygen through a Fenton-like reaction, leading to an enhanced PDT under the irradiation of light. More importantly, the process of catalyzing H2O2 decomposition at the tumor location can also generate a cytotoxic hydroxyl radical (˙OH), achieving an excellent chemodynamic therapy (CDT) to enhance the ROS mediated anti-cancer effect. Notably, the nano-enzyme exerts a high loading content of the photosensitizer, which minimizes the side effects probably caused by the vector.
中文翻译:

一种光和肿瘤微环境激活的纳米酶,具有增强的 ROS 生成和缺氧缓解,用于有效的癌症治疗
活性氧(ROS)介导的肿瘤治疗策略具有广阔的前景并引起越来越多的关注,其中光动力疗法(PDT)已得到广泛认可。然而,PDT的抗癌作用受到缺氧肿瘤微环境(TME)的极大限制。因此,探索一种可以缓解肿瘤缺氧的治疗策略被认为是克服这一问题的关键。在此,我们开发了一种新型纳米酶 (MnO 2 @TPP-PEG),可以准确地进行 H 2 O 2的肿瘤特异性催化,通过类芬顿反应产生氧气,从而在光照射下增强 PDT . 更重要的是,催化H 2 O 2的过程在肿瘤部位分解还可以产生具有细胞毒性的羟基自由基(˙OH),从而实现出色的化学动力学疗法(CDT),以增强ROS介导的抗癌作用。值得注意的是,纳米酶发挥了高负载量的光敏剂,这最大限度地减少了可能由载体引起的副作用。
更新日期:2021-09-13
中文翻译:

一种光和肿瘤微环境激活的纳米酶,具有增强的 ROS 生成和缺氧缓解,用于有效的癌症治疗
活性氧(ROS)介导的肿瘤治疗策略具有广阔的前景并引起越来越多的关注,其中光动力疗法(PDT)已得到广泛认可。然而,PDT的抗癌作用受到缺氧肿瘤微环境(TME)的极大限制。因此,探索一种可以缓解肿瘤缺氧的治疗策略被认为是克服这一问题的关键。在此,我们开发了一种新型纳米酶 (MnO 2 @TPP-PEG),可以准确地进行 H 2 O 2的肿瘤特异性催化,通过类芬顿反应产生氧气,从而在光照射下增强 PDT . 更重要的是,催化H 2 O 2的过程在肿瘤部位分解还可以产生具有细胞毒性的羟基自由基(˙OH),从而实现出色的化学动力学疗法(CDT),以增强ROS介导的抗癌作用。值得注意的是,纳米酶发挥了高负载量的光敏剂,这最大限度地减少了可能由载体引起的副作用。

























 京公网安备 11010802027423号
京公网安备 11010802027423号