Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2021-09-13 , DOI: 10.1016/j.bmc.2021.116387 Srinivas Chamakuri 1 , Mee-Kyung Chung 1 , Errol L G Samuel 2 , Kevin A Tran 1 , Ying-Chu Chen 1 , Pranavanand Nyshadham 1 , Conrad Santini 1 , Martin M Matzuk 3 , Damian W Young 4
|
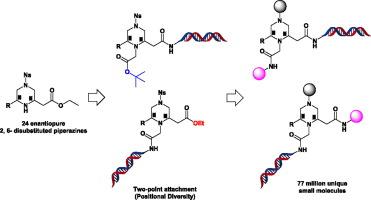
Here we report the successful construction of a novel, stereochemically diverse DNA-Encoded Chemical Library (DECL) by utilizing 24 enantiomerically pure trifunctional 2, 6- di-substituted piperazines as central cores. We introduce the concept of positional diversity by placing the DNA attachment at either of two possible sites on the piperazine scaffold. Using a wide range of building blocks, a diverse library of 77 million compounds was produced. Cheminformatic analysis demonstrates that this library occupies a wide swath of chemical space, and that the piperazine scaffolds confers different shape diversity compared to the commonly used triazine core.
中文翻译:

立体化学多样性哌嗪 DNA 编码化学文库的设计与构建
在这里,我们报告了通过利用 24 个对映体纯的三官能 2, 6-二取代哌嗪作为中心核心,成功构建了一个新颖的、立体化学多样化的 DNA 编码化学文库 (DECL)。我们通过将 DNA 附件放置在哌嗪支架上的两个可能位点中的任一个来引入位置多样性的概念。使用广泛的构建块,生成了一个包含 7700 万种化合物的多样化库。化学信息学分析表明,该文库占据了广泛的化学空间,与常用的三嗪核心相比,哌嗪支架具有不同的形状多样性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号