当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sequence Determines the Switch in the Fibril Forming Regions in the Low-Complexity FUS Protein and Its Variants
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpclett.1c02310 Abhinaw Kumar 1 , Debayan Chakraborty 1 , Mauro Lorenzo Mugnai 1 , John E Straub 2 , D Thirumalai 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.jpclett.1c02310 Abhinaw Kumar 1 , Debayan Chakraborty 1 , Mauro Lorenzo Mugnai 1 , John E Straub 2 , D Thirumalai 1
Affiliation
|
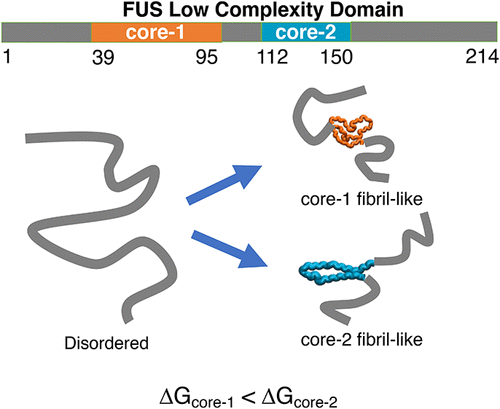
Residues spanning distinct regions of the low-complexity domain of the RNA-binding protein, Fused in Sarcoma (FUS-LC), form fibril structures with different core morphologies. Solid-state NMR experiments show that the 214-residue FUS-LC forms a fibril with an S-bend (core-1, residues 39–95), while the rest of the protein is disordered. In contrast, the fibrils of the C-terminal variant (FUS-LC-C; residues 111–214) have a U-bend topology (core-2, residues 112–150). Absence of the U-bend in FUS-LC implies that the two fibril cores do not coexist. Computer simulations show that these perplexing findings could be understood in terms of the population of sparsely populated fibril-like excited states in the monomer. The propensity to form core-1 is higher compared to core-2. We predict that core-2 forms only in truncated variants that do not contain the core-1 sequence. At the monomer level, sequence-dependent enthalpic effects determine the relative stabilities of the core-1 and core-2 topologies.
中文翻译:

序列确定低复杂性 FUS 蛋白及其变体中原纤维形成区域的开关
跨越肉瘤融合蛋白 (FUS-LC) 的低复杂性结构域的不同区域的残基形成具有不同核心形态的原纤维结构。固态 NMR 实验表明,214 个残基 FUS-LC 形成具有 S 型弯曲的原纤维(核心 1,残基 39-95),而蛋白质的其余部分是无序的。相反,C 末端变体(FUS-LC-C;残基 111-214)的原纤维具有 U 形弯曲拓扑结构(核心 2,残基 112-150)。FUS-LC 中不存在 U 形弯曲意味着两个原纤维核不共存。计算机模拟表明,这些令人困惑的发现可以根据单体中稀疏分布的原纤维状激发态的数量来理解。与 core-2 相比,形成 core-1 的倾向更高。我们预测 core-2 仅在不包含 core-1 序列的截短变体中形成。在单体水平上,依赖于序列的焓效应决定了 core-1 和 core-2 拓扑的相对稳定性。
更新日期:2021-09-23
中文翻译:

序列确定低复杂性 FUS 蛋白及其变体中原纤维形成区域的开关
跨越肉瘤融合蛋白 (FUS-LC) 的低复杂性结构域的不同区域的残基形成具有不同核心形态的原纤维结构。固态 NMR 实验表明,214 个残基 FUS-LC 形成具有 S 型弯曲的原纤维(核心 1,残基 39-95),而蛋白质的其余部分是无序的。相反,C 末端变体(FUS-LC-C;残基 111-214)的原纤维具有 U 形弯曲拓扑结构(核心 2,残基 112-150)。FUS-LC 中不存在 U 形弯曲意味着两个原纤维核不共存。计算机模拟表明,这些令人困惑的发现可以根据单体中稀疏分布的原纤维状激发态的数量来理解。与 core-2 相比,形成 core-1 的倾向更高。我们预测 core-2 仅在不包含 core-1 序列的截短变体中形成。在单体水平上,依赖于序列的焓效应决定了 core-1 和 core-2 拓扑的相对稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号