当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sulfonyl-Promoted Michaelis–Arbuzov-Type Reaction: An Approach to S/Se–P Bonds
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01681 Suhail A Rather 1, 2 , Mohammad Yaqoob Bhat 1, 2 , Feroze Hussain 1, 2 , Qazi Naveed Ahmed 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01681 Suhail A Rather 1, 2 , Mohammad Yaqoob Bhat 1, 2 , Feroze Hussain 1, 2 , Qazi Naveed Ahmed 1, 2
Affiliation
|
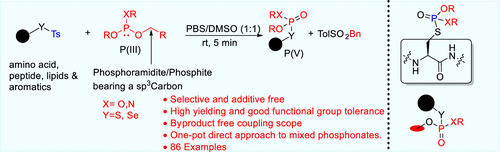
By facilitating the chemical conversion of thiols to thiosulfonates, phosphoramidite/phosphite bearing sp3-hybridized carbon serves as an ideal coupling material to forge new connections at room temperature. In this work, a functional group-induced, additive-free, novel, S–P bond-forming approach is presented. This protocol exhibits good functional group tolerance with wide applications that include phosphorylation of cysteine derivatives, development of a one-pot approach to mixed unsymmetrical thiophosphonates, and extension of the concept to different Se–P bonds. Meticulously, our reaction also generated a S–P bond against cyclic 1,2-dithiane-1-dioxide in a byproduct-free manner. These Michaelis–Arbuzov-type reactions are easy to conduct, work efficiently in a reduced reaction time, and are applicable to gram-scale preparation as well.
中文翻译:

磺酰基促进的 Michaelis-Arbuzov 型反应:S/Se-P 键的一种方法
通过促进硫醇向硫代磺酸盐、亚磷酰胺/亚磷酸盐的化学转化,sp 3- 杂化碳是一种理想的偶联材料,可在室温下形成新的连接。在这项工作中,提出了一种官能团诱导的、无添加剂的、新颖的 S-P 键形成方法。该协议表现出良好的官能团耐受性,具有广泛的应用,包括半胱氨酸衍生物的磷酸化、混合不对称硫代膦酸盐的一锅法的开发,以及将概念扩展到不同的 Se-P 键。精心地,我们的反应还以无副产物的方式生成了针对环状 1,2-二噻烷-1-二氧化物的 S-P 键。这些 Michaelis-Arbuzov 型反应易于进行,在缩短的反应时间内高效工作,也适用于克级制备。
更新日期:2021-10-01
中文翻译:

磺酰基促进的 Michaelis-Arbuzov 型反应:S/Se-P 键的一种方法
通过促进硫醇向硫代磺酸盐、亚磷酰胺/亚磷酸盐的化学转化,sp 3- 杂化碳是一种理想的偶联材料,可在室温下形成新的连接。在这项工作中,提出了一种官能团诱导的、无添加剂的、新颖的 S-P 键形成方法。该协议表现出良好的官能团耐受性,具有广泛的应用,包括半胱氨酸衍生物的磷酸化、混合不对称硫代膦酸盐的一锅法的开发,以及将概念扩展到不同的 Se-P 键。精心地,我们的反应还以无副产物的方式生成了针对环状 1,2-二噻烷-1-二氧化物的 S-P 键。这些 Michaelis-Arbuzov 型反应易于进行,在缩短的反应时间内高效工作,也适用于克级制备。



























 京公网安备 11010802027423号
京公网安备 11010802027423号