当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
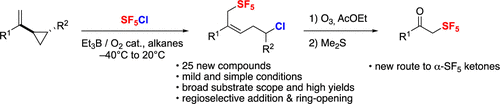
Radical 1,5-Chloropentafluorosulfanylation of Unactivated Vinylcyclopropanes and Transformation into α-SF5 Ketones
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01886 Fang-Fang Feng 1, 2 , Jun-An Ma 2 , Dominique Cahard 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.joc.1c01886 Fang-Fang Feng 1, 2 , Jun-An Ma 2 , Dominique Cahard 1
Affiliation
|
The radical 1,5-chloropentafluorosulfanylation of vinyl cyclopropanes (VCPs) initiated by Et3B/O2 affords allylic pentafluorosulfanyl/homoallylic chloride products through the ring-strain release of the cyclopropane. The VCP substitution pattern was investigated. The utility of this reaction was illustrated in post-transformation of the C═C bond by ozonolysis, giving access to valuable α-SF5 carbonyl compounds.
中文翻译:

未活化乙烯基环丙烷的自由基 1,5-氯五氟磺基化和转化为 α-SF5 酮
由 Et 3 B/O 2引发的乙烯基环丙烷 (VCP) 的自由基 1,5-氯五氟硫烷基化通过环丙烷的环应变释放提供烯丙基五氟硫烷基/高烯丙基氯产物。研究了 VCP 取代模式。该反应的效用在通过臭氧分解 C=C 键的后转化中得到了说明,从而获得了有价值的 α-SF 5羰基化合物。
更新日期:2021-10-01
中文翻译:

未活化乙烯基环丙烷的自由基 1,5-氯五氟磺基化和转化为 α-SF5 酮
由 Et 3 B/O 2引发的乙烯基环丙烷 (VCP) 的自由基 1,5-氯五氟硫烷基化通过环丙烷的环应变释放提供烯丙基五氟硫烷基/高烯丙基氯产物。研究了 VCP 取代模式。该反应的效用在通过臭氧分解 C=C 键的后转化中得到了说明,从而获得了有价值的 α-SF 5羰基化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号