当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of a Nitro-Forming Enzyme Involved in Fosfazinomycin Biosynthesis
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.biochem.1c00512 Hannah Valentino 1, 2 , Pablo Sobrado 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-13 , DOI: 10.1021/acs.biochem.1c00512 Hannah Valentino 1, 2 , Pablo Sobrado 1, 2
Affiliation
|
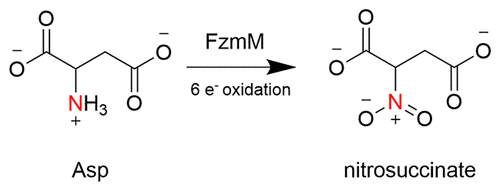
N-hydroxylating monooxygenases (NMOs) are a subclass of flavin-dependent enzymes that hydroxylate nitrogen atoms. Recently, unique NMOs that perform multiple reactions on one substrate molecule have been identified. Fosfazinomycin M (FzmM) is one such NMO, forming nitrosuccinate from aspartate (Asp) in the fosfazinomycin biosynthetic pathway in some Streptomyces sp. This work details the biochemical and kinetic analysis of FzmM. Steady-state kinetic investigation shows that FzmM performs a coupled reaction with Asp (kcat, 3.0 ± 0.01 s–1) forming nitrosuccinate, which can be converted to fumarate and nitrite by the action of FzmL. FzmM displays a 70-fold higher kcat/KM value for NADPH compared to NADH and has a narrow optimal pH range (7.5–8.0). Contrary to other NMOs where the kred is rate-limiting, FzmM exhibits a very fast kred (50 ± 0.01 s–1 at 4 °C) with NADPH. NADPH binds at a KD value of ∼400 μM, and hydride transfer occurs with pro-R stereochemistry. Oxidation of FzmM in the absence of Asp exhibits a spectrum with a shoulder at ∼370 nm, consistent with the formation of a C(4a)-hydroperoxyflavin intermediate, which decays into oxidized flavin and hydrogen peroxide at a rate 100-fold slower than the kcat. This reaction is enhanced in the presence of Asp with a slightly faster kox than the kcat, suggesting that flavin dehydration or Asp oxidation is partially rate limiting. Multiple sequence analyses of FzmM to NMOs identified conserved residues involved in flavin binding but not for NADPH. Additional sequence analysis to related monooxygenases suggests that FzmM shares sequence motifs absent in other NMOs.
中文翻译:

参与磷霉素生物合成的硝基形成酶的表征
N-羟基化单加氧酶 (NMO) 是使氮原子羟基化的黄素依赖性酶的一个子类。最近,已鉴定出对一个底物分子进行多重反应的独特 NMO。Fosfazinomycin M (FzmM) 是一种这样的 NMO,在某些链霉菌属的 fosfazinomycin 生物合成途径中从天冬氨酸 (Asp) 形成亚硝基琥珀酸盐。这项工作详细介绍了 FzmM 的生化和动力学分析。稳态动力学研究表明,FzmM 与 Asp ( k cat , 3.0 ± 0.01 s –1 )发生偶联反应,形成亚硝基琥珀酸盐,后者在 FzmL 的作用下可转化为富马酸盐和亚硝酸盐。FzmM 显示出高 70 倍的k cat / K M与 NADH 相比,NADPH 的值较小,并且具有较窄的最佳 pH 值范围 (7.5-8.0)。与k红限速的其他 NMO 不同,FzmM与 NADPH显示出非常快的k红(4 °C 时为50 ± 0.01 s –1)。NADPH 结合的K D值约为 400 μM,氢化物转移发生在pro-R立体化学中。在没有 Asp 的情况下,FzmM 的氧化显示出在~370 nm 处具有肩峰的光谱,这与 C(4a)-氢过氧黄素中间体的形成一致,其衰变成氧化黄素和过氧化氢的速度比ķ猫。该反应在 Asp 存在下增强,速度稍快k ox比k cat 高,表明黄素脱水或 Asp 氧化是部分限速的。FzmM 到 NMO 的多重序列分析确定了参与黄素结合但不参与 NADPH 的保守残基。对相关单加氧酶的额外序列分析表明 FzmM 共享其他 NMO 中不存在的序列基序。
更新日期:2021-09-28
中文翻译:

参与磷霉素生物合成的硝基形成酶的表征
N-羟基化单加氧酶 (NMO) 是使氮原子羟基化的黄素依赖性酶的一个子类。最近,已鉴定出对一个底物分子进行多重反应的独特 NMO。Fosfazinomycin M (FzmM) 是一种这样的 NMO,在某些链霉菌属的 fosfazinomycin 生物合成途径中从天冬氨酸 (Asp) 形成亚硝基琥珀酸盐。这项工作详细介绍了 FzmM 的生化和动力学分析。稳态动力学研究表明,FzmM 与 Asp ( k cat , 3.0 ± 0.01 s –1 )发生偶联反应,形成亚硝基琥珀酸盐,后者在 FzmL 的作用下可转化为富马酸盐和亚硝酸盐。FzmM 显示出高 70 倍的k cat / K M与 NADH 相比,NADPH 的值较小,并且具有较窄的最佳 pH 值范围 (7.5-8.0)。与k红限速的其他 NMO 不同,FzmM与 NADPH显示出非常快的k红(4 °C 时为50 ± 0.01 s –1)。NADPH 结合的K D值约为 400 μM,氢化物转移发生在pro-R立体化学中。在没有 Asp 的情况下,FzmM 的氧化显示出在~370 nm 处具有肩峰的光谱,这与 C(4a)-氢过氧黄素中间体的形成一致,其衰变成氧化黄素和过氧化氢的速度比ķ猫。该反应在 Asp 存在下增强,速度稍快k ox比k cat 高,表明黄素脱水或 Asp 氧化是部分限速的。FzmM 到 NMO 的多重序列分析确定了参与黄素结合但不参与 NADPH 的保守残基。对相关单加氧酶的额外序列分析表明 FzmM 共享其他 NMO 中不存在的序列基序。


























 京公网安备 11010802027423号
京公网安备 11010802027423号