Journal of King Saud University-Science ( IF 3.8 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.jksus.2021.101602 Tzu-Yu Pan , Wei-Chung Tsai , Chun-Hsiang Tan , Ching-Mei Cheng , Wei Chen , Thiagarajan Soundappan , Mariadhas Valan Arasu , Naif Abdullah Al-Dhabi , Chia-Fang Wu , Vinoth Kumar Ponnusamy , Ming-Tsang Wu
|
Objectives
There is no analytical method for simultaneous monitoring of warfarin and novel direct oral anticoagulants (NOACs) in human urine samples in a single run in the clinics. Although several studies have reported their measurements in human blood samples, but its sample collection is more invasive and time-consuming, thereby challenging to monitor frequently.
Methods
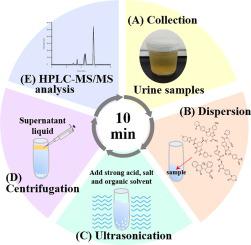
In this work, we developed a fast microextraction technique (ultrasound-assisted salt-induced liquid-liquid microextraction, USA-SI-LLME) coupled with high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS) to rapidly quantify four commonly used NOACs drugs (apixaban, dabigatran, edoxaban, and rivaroxaban) and warfarin in human urine samples. USA-SI-LLME conditions were optimized using a water-miscible organic solvent as an extraction solvent, high salt concentrations, sample pH, and extraction time (∼5.5 min).
Results and conclusions
The analytical method showed excellent linearities from 0.5 to 500 μg/L for apixaban, edoxaban, rivaroxaban, warfarin, and 1 ∼ 500 μg/L for dabigatran. Intra- and inter-day precision values were <9.31% and R2 > 0.99 for all analytes. Limits of detection ranged between 0.07 ∼ 0.18 μg/L, and relative recoveries ranged between 92.18 and 110.15%. This method was successfully applied to analyze 15 one-spot urine samples from 15 clinical patients who regularly took warfarin or NOACs, and high accuracy was found. We concluded that this method could be used as a non-invasive high-throughput and rapid monitoring of NOACs and warfarin in human urine in clinical settings and could provide timely analysis during emergency care.
中文翻译:

使用绿色微萃取技术结合 LC-MS/MS 快速同时临床监测人尿中的五种口服抗凝药物
目标
目前还没有一种分析方法可以在诊所的单次运行中同时监测人尿样本中的华法林和新型直接口服抗凝剂 (NOAC)。尽管有几项研究报告了他们在人体血液样本中的测量结果,但其样本采集更具侵入性和耗时,因此难以频繁监测。
方法
在这项工作中,我们开发了一种快速微萃取技术(超声辅助盐诱导液-液微萃取,USA-SI-LLME)结合高效液相色谱-串联质谱(HPLC-MS/MS)快速定量四种人尿样本中常用的 NOACs 药物(阿哌沙班、达比加群、依度沙班和利伐沙班)和华法林。使用与水混溶的有机溶剂作为萃取溶剂、高盐浓度、样品 pH 值和萃取时间(约 5.5 分钟)优化了 USA-SI-LLME 条件。
结果和结论
分析方法显示,阿哌沙班、依度沙班、利伐沙班、华法林在 0.5 至 500 μg/L 范围内具有出色的线性,而达比加群在 1 ∼ 500 μg/L 范围内线性良好。 所有分析物的日内和日间精密度值<9.31%,R 2 > 0.99。检测限范围在 0.07 ∼ 0.18 μg/L 之间,相对回收率介于 92.18 和 110.15% 之间。将该方法成功应用于15例定期服用华法林或NOACs的临床患者的15份单点尿样分析,结果准确度高。我们得出的结论是,该方法可用作临床环境中人尿中 NOAC 和华法林的无创高通量快速监测,并可在紧急护理期间提供及时分析。


























 京公网安备 11010802027423号
京公网安备 11010802027423号