当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold. A new potential non-classical isostere of indole and a precursor of push–pull dyes
Chemical Science ( IF 8.4 ) Pub Date : 2021-08-30 , DOI: 10.1039/d1sc04155j Kuno Schwärzer 1 , Saroj K Rout 1 , Derya Bessinger 1 , Fabio Lima 2 , Cara E Brocklehurst 2 , Konstantin Karaghiosoff 1 , Thomas Bein 1 , Paul Knochel 1
Chemical Science ( IF 8.4 ) Pub Date : 2021-08-30 , DOI: 10.1039/d1sc04155j Kuno Schwärzer 1 , Saroj K Rout 1 , Derya Bessinger 1 , Fabio Lima 2 , Cara E Brocklehurst 2 , Konstantin Karaghiosoff 1 , Thomas Bein 1 , Paul Knochel 1
Affiliation
|
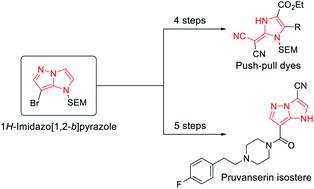
We report the selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold using a Br/Mg-exchange, as well as regioselective magnesiations and zincations with TMP-bases (TMP = 2,2,6,6-tetramethylpiperidyl), followed by trapping reactions with various electrophiles. In addition, we report a fragmentation of the pyrazole ring, giving access to push–pull dyes with a proaromatic (1,3-dihydro-2H-imidazol-2-ylidene)malononitrile core. These functionalization methods were used in the synthesis of an isostere of the indolyl drug pruvanserin. Comparative assays between the original drug and the isostere showed that a substitution of the indole ring with a 1H-imidazo[1,2-b]pyrazole results in a significantly improved solubility in aqueous media.
中文翻译:

1H-咪唑并[1,2-b]吡唑支架的选择性功能化。吲哚的一种新的潜在非经典等排体和推拉染料的前体
我们报告了 1 H-咪唑并[1,2- b ]吡唑支架的选择性官能化使用 Br/Mg 交换,以及区域选择性氧化和 TMP 碱(TMP = 2,2,6,6-四甲基哌啶基),然后与各种亲电子试剂发生捕获反应。此外,我们报告了吡唑环的断裂,从而获得了具有前芳香族(1,3-二氢-2 H-咪唑-2-亚基)丙二腈核的推拉染料。这些功能化方法用于合成吲哚基药物普万色林的等排体。原始药物和等排体之间的比较分析表明,吲哚环被 1 H -咪唑并[1,2- b]吡唑显着提高了在水性介质中的溶解度。
更新日期:2021-09-12
中文翻译:

1H-咪唑并[1,2-b]吡唑支架的选择性功能化。吲哚的一种新的潜在非经典等排体和推拉染料的前体
我们报告了 1 H-咪唑并[1,2- b ]吡唑支架的选择性官能化使用 Br/Mg 交换,以及区域选择性氧化和 TMP 碱(TMP = 2,2,6,6-四甲基哌啶基),然后与各种亲电子试剂发生捕获反应。此外,我们报告了吡唑环的断裂,从而获得了具有前芳香族(1,3-二氢-2 H-咪唑-2-亚基)丙二腈核的推拉染料。这些功能化方法用于合成吲哚基药物普万色林的等排体。原始药物和等排体之间的比较分析表明,吲哚环被 1 H -咪唑并[1,2- b]吡唑显着提高了在水性介质中的溶解度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号