Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.bioorg.2021.105349 Surendra Kunwar 1 , Soo-Yeon Hwang 2 , Pramila Katila 1 , Minjung Seo 2 , Tara Man Kadayat 1 , Youngjoo Kwon 2 , Eung-Seok Lee 1
|
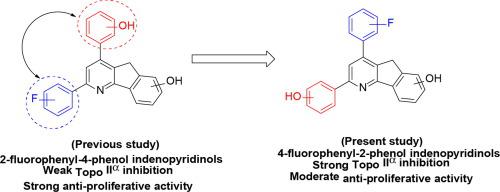
A series of fluorinated and hydroxylated 2,4-diphenyl indenopyridinols were designed and synthesized using l-proline-catalyzed and microwave-assisted synthetic methods for the development of new anticancer agents. Adriamycin and etoposide were used as reference compounds for the evaluation of topo IIα inhibitory and anti-proliferative activity of the synthesized compounds. Exploring the structure–activity relationships of 36 prepared compounds and biological results, most of the compounds with ortho- and para-fluorophenyl at 4-position of indenopyridinol ring displayed strong topo IIα inhibition. In addition, the majority of the ortho- and meta-fluorophenyl substituted compounds 1–24 displayed strong anti-proliferative activity against DU145 prostate cancer cell line compared to the positive controls. Interestingly, compound 4 possessing ortho-phenolic and ortho-fluorophenyl group at 2- and 4-position, respectively of the central pyridine ring showed high anti-proliferative activity (IC50 = 0.82 μM) against T47D human breast cancer cell line, while para-phenolic and para-fluorophenyl substituted compound 36 exhibited potent topo IIα inhibitory activity with 94.7% and 88.6% inhibition at 100 μM and 20 μM concentration, respectively. A systematic comparison between the results of this study and the previous study indicated that minor changes in the position of functional groups in the structure affect the topo IIα inhibitory activity and anti-proliferative activity of the compounds. The findings from this study will provide valuable information to the researchers working on the medicinal chemistry of topoisomerase IIα-targeted anticancer agents.
中文翻译:

具有增强的拓扑异构酶 IIα 抑制活性的 4-氟苯基取代的 5H-茚并 [1,2-b] 吡啶醇:合成、生物学评价和结构-活性关系
使用l-脯氨酸催化和微波辅助合成方法设计和合成了一系列氟化和羟基化的 2,4-二苯基茚并吡啶醇,用于开发新的抗癌剂。阿霉素和依托泊苷被用作参考化合物,用于评估合成化合物的拓扑 IIα 抑制和抗增殖活性。探索制备的 36 种化合物的构效关系和生物学结果,大多数在茚并吡啶环的 4-位具有邻-和对-氟苯基的化合物表现出强烈的拓扑 IIα 抑制。此外,大多数邻氟苯基和间氟苯基取代的化合物1 – 24与阳性对照相比,对 DU145 前列腺癌细胞系显示出强烈的抗增殖活性。有趣的是,在中央吡啶环的 2-位和 4-位分别具有邻苯酚和邻氟苯基的化合物4 对 T47D 人乳腺癌细胞系显示出高抗增殖活性(IC 50 = 0.82 μM),而对-酚类和对-氟苯基取代的化合物36在 100 μM 和 20 μM 浓度下,分别表现出有效的 topo IIα 抑制活性,抑制率分别为 94.7% 和 88.6%。本研究结果与以往研究结果的系统比较表明,结构中官能团位置的微小变化会影响化合物的拓扑IIα抑制活性和抗增殖活性。这项研究的结果将为研究拓扑异构酶 IIα 靶向抗癌药物的药物化学的研究人员提供有价值的信息。


























 京公网安备 11010802027423号
京公网安备 11010802027423号