Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.bioorg.2021.105353 Tao Zhang 1 , Zhongxia Zhou 1 , Waleed A Zalloum 2 , Zhao Wang 1 , Zhipeng Fu 1 , Srinivasulu Cherukupalli 1 , Da Feng 1 , Yanying Sun 1 , Shenghua Gao 1 , Erik De Clercq 3 , Christophe Pannecouque 3 , Dongwei Kang 4 , Peng Zhan 4 , Xinyong Liu 4
|
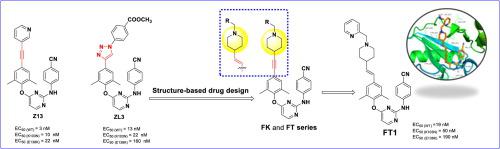
Herein, alkenylpiperidine and alkynylpiperidine moieties were introduced into the left wing of DAPYs (diarylpyrimidines) to explore the new site of the NNIBP (non-nucleoside inhibitor binding pocket) protein-solvent interface region via the structure-based drug design strategy. All the synthesized compounds displayed nanomolar to submicromolar activity against WT (wild-type) HIV-1. Among all, compound FT1 (EC50 = 19 nM) was found to be the most active molecule, which is better than NVP (EC50 = 0.10 μM). In addition, most of the compounds displayed micromolar activity against K103N and E138K mutant strains, while FT1 (EC50(K103N) = 50 nM, EC50(E138K) = 0.19 µM) still has the most effective activity. The molecular dynamics simulation studies revealed that the presence of pyridine moiety of FT1 was essential and played a significant role in its binding with RT (reverse transcriptase).
中文翻译:

通过探索 NNIBP 的疏水通道,设计、合成和抗病毒评估新型哌啶取代的芳基嘧啶作为 HIV-1 NNRTIs
在此,烯基哌啶和炔基哌啶部分被引入 DAPYs(二芳基嘧啶)的左翼,通过基于结构的药物设计策略探索 NNIBP(非核苷抑制剂结合口袋)蛋白质-溶剂界面区域的新位点。所有合成的化合物对 WT(野生型)HIV-1 都显示出纳摩尔到亚微摩尔的活性。其中,化合物FT1 (EC 50 = 19 nM) 是活性最强的分子,优于 NVP (EC 50 = 0.10 μM)。此外,大多数化合物对 K103N 和 E138K 突变株表现出微摩尔活性,而FT1 (EC 50(K103N) = 50 nM,EC50(E138K) = 0.19 µM) 仍然具有最有效的活性。分子动力学模拟研究表明,FT1的吡啶部分的存在是必不可少的,并且在其与 RT(逆转录酶)的结合中发挥了重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号