Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2021-09-11 , DOI: 10.1016/j.bmcl.2021.128356 Min Lv 1 , Qianjun Ma 1 , Shaoyong Zhang 2 , Hui Xu 1
|
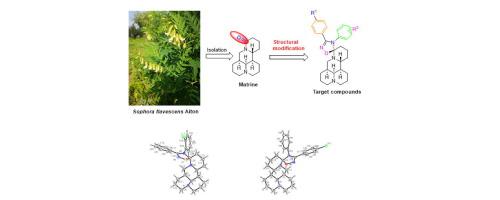
In order to increase the agricultural properties of matrine, a series of novel matrine-type alkaloids containing spiro-1,2,4-oxadiazoline fragment at the C-15 position were prepared. Eight target molecules were elucidated by X-ray single-crystal diffraction. The antifeedant activities of Ig and IIIh against Mythimna separata Walker were>1.7 folds of the precursor matrine. The acaricidal activities of Ij, IIe, IIg, IIi and IIIa against Tetranychus cinnabarinus Boisduval were 2.6–3.7 folds of matrine. Especially IIg (R1 = R2 = 4-Cl) and IIi (R1 = 4-Cl; R2 = 4-Br) exhibited the pronounced antifeedant and acaricidal activities. SARs showed that their pesticidal activities were related to the substitutents and their positions on the phenyl rings at the C-3 and N-4 positions of 1,2,4-oxadiazoline skeleton.
中文翻译:

螺1,2,4-恶二唑啉-稠合苦参型生物碱杀虫剂的构建
为了增加苦参碱的农用性,制备了一系列 C-15位含有螺-1,2,4-恶二唑啉片段的苦参类生物碱。通过 X 射线单晶衍射阐明了八个目标分子。Ig和IIIh对Mythimna separata Walker的拒食活性是前体苦参碱的1.7倍以上。的杀螨活性IJ,IIe上,IIG,III和IIIa受体对朱砂叶螨分别为2.6 -苦参碱的3.7倍。特别是IIg (R 1 = R 2 = 4-Cl) 和IIi (R 1 = 4-Cl; R 2 = 4-Br) 表现出明显的拒食和杀螨活性。SARs表明,它们的杀虫活性与1,2,4-恶二唑啉骨架C-3和N-4位苯环上的取代基及其位置有关。


























 京公网安备 11010802027423号
京公网安备 11010802027423号