当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Mechanistic Insights of the Formation of 3-Hydroxyquinolin-2-ones including Viridicatin from 2-Chloro-N,3-diaryloxirane-2-carboxamides under Acid-Catalyzed Rearrangements
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.joc.1c01592 Vakhid A Mamedov 1 , Vera L Mamedova 1 , Zheng-Wang Qu 2 , Hui Zhu 2 , Venera R Galimullina 1 , Dmitry E Korshin 1 , Gul'naz Z Khikmatova 1 , Igor A Litvinov 1 , Shamil K Latypov 1 , Oleg G Sinyashin 1 , Stefan Grimme 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.joc.1c01592 Vakhid A Mamedov 1 , Vera L Mamedova 1 , Zheng-Wang Qu 2 , Hui Zhu 2 , Venera R Galimullina 1 , Dmitry E Korshin 1 , Gul'naz Z Khikmatova 1 , Igor A Litvinov 1 , Shamil K Latypov 1 , Oleg G Sinyashin 1 , Stefan Grimme 2
Affiliation
|
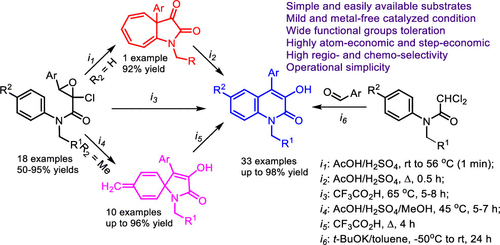
N-Benzyl-2-chloro-N,3-diaryloxirane-2-carboxamides, easily obtained from aromatic aldehydes and anilides of dichloroacetic acid under Darzens condensation conditions, proved to be excellent starting compounds for the synthesis of 3-hydroxyindolin-2-ones, cyclohepto[b]pyrrole-2,3-diones, and 1-azaspiro[4.5]deca-3,6,9-triene-2-ones via the C(sp2)–C(sp2) bond formation in the first case and C(sp2)–C(sp3) bond formation in the second and third cases. Under optimized reaction conditions, 3-hydroxyindolin-2-ones are obtained in a one-pot process, which involves the treatment of N-benzyl-2-chloro-N,3-diaryloxirane-2-carboxamides with CF3CO2H or AcOH/H2SO4. In the case of intramolecular cyclization, the detailed reaction channels depend strongly on the substituents present in the anilide component and in the aromatic ring of the aldehyde component of N-benzyl-2-chloro-N,3-diaryloxirane-2-carboxamides, as well as the temperature and duration of the reaction. A combined experimental and DFT mechanistic study of the formation of 1-benzyl-3-hydroxy-4-arylquinolin-2(1H)-ones showed that there are three competing reaction channels: (a) ring-closure via the ipso site, (b) ring-closure via the 1,2-Cl shift, and (c) ring-closure via the ortho site. Such mechanistic insights enabled an effective one-pot gram-scale synthesis of viridicatin from benzaldehyde and 2,2-dichloro-N-(4-methoxybenzyl)-N-phenylacetamide.
中文翻译:

在酸催化重排下从 2-Chloro-N,3-diaryloxirane-2-carboxamides 形成 3-Hydroxyquinolin-2-ones 包括 Viridicatin 的合成和机理见解
N -Benzyl-2-chloro - N ,3-diaryloxirane-2-carboxamides 在 Darzens 缩合条件下很容易从二氯乙酸的芳香醛和苯胺中获得,被证明是合成 3-羟基吲哚-2-酮的优良起始化合物, cyclohepto[ b ]pyrrole-2,3-diones 和 1-azaspiro[4.5]deca-3,6,9-triene-2-ones 通过 C(sp 2 )–C(sp 2 ) 键形成第一种情况和 C(sp 2 )–C(sp 3 ) 键在第二种和第三种情况下的形成。在优化的反应条件下,一锅法得到3-羟基吲哚-2-酮,其中包括N-苄基-2-氯-N的处理,3-二芳基环氧乙烷-2-甲酰胺与CF 3 CO 2 H 或AcOH/H 2 SO 4。在分子内环化的情况下,详细的反应通道强烈依赖于存在于苯胺组分和N-苄基-2-氯-N ,3-二芳基环氧乙烷-2-甲酰胺的醛组分的芳环中的取代基,如以及反应的温度和持续时间。1-benzyl-3-hydroxy-4-arylquinolin-2(1 H ) -ones 形成的联合实验和 DFT 机理研究表明存在三个竞争反应通道:(a) 通过ipso闭环位点,(b)通过 1,2-Cl 转变的闭环,和(c)通过邻位的闭环。这样的机理见解使从苯甲醛和 2,2-二氯-N- (4-甲氧基苄基) -N-苯基乙酰胺有效地一锅克级合成绿藻素成为可能。
更新日期:2021-10-01
中文翻译:

在酸催化重排下从 2-Chloro-N,3-diaryloxirane-2-carboxamides 形成 3-Hydroxyquinolin-2-ones 包括 Viridicatin 的合成和机理见解
N -Benzyl-2-chloro - N ,3-diaryloxirane-2-carboxamides 在 Darzens 缩合条件下很容易从二氯乙酸的芳香醛和苯胺中获得,被证明是合成 3-羟基吲哚-2-酮的优良起始化合物, cyclohepto[ b ]pyrrole-2,3-diones 和 1-azaspiro[4.5]deca-3,6,9-triene-2-ones 通过 C(sp 2 )–C(sp 2 ) 键形成第一种情况和 C(sp 2 )–C(sp 3 ) 键在第二种和第三种情况下的形成。在优化的反应条件下,一锅法得到3-羟基吲哚-2-酮,其中包括N-苄基-2-氯-N的处理,3-二芳基环氧乙烷-2-甲酰胺与CF 3 CO 2 H 或AcOH/H 2 SO 4。在分子内环化的情况下,详细的反应通道强烈依赖于存在于苯胺组分和N-苄基-2-氯-N ,3-二芳基环氧乙烷-2-甲酰胺的醛组分的芳环中的取代基,如以及反应的温度和持续时间。1-benzyl-3-hydroxy-4-arylquinolin-2(1 H ) -ones 形成的联合实验和 DFT 机理研究表明存在三个竞争反应通道:(a) 通过ipso闭环位点,(b)通过 1,2-Cl 转变的闭环,和(c)通过邻位的闭环。这样的机理见解使从苯甲醛和 2,2-二氯-N- (4-甲氧基苄基) -N-苯基乙酰胺有效地一锅克级合成绿藻素成为可能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号