当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Chromeno[4,3,2-de] [1,6]naphthyridine Derivatives via Pseudo Four-Component Reaction
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-09 , DOI: 10.1002/slct.202101962 Jianbo Gan 1 , Naili Luo 1 , Chengjun Wu 1 , Xinyi Wan 1 , Cunde Wang 1
ChemistrySelect ( IF 2.1 ) Pub Date : 2021-09-09 , DOI: 10.1002/slct.202101962 Jianbo Gan 1 , Naili Luo 1 , Chengjun Wu 1 , Xinyi Wan 1 , Cunde Wang 1
Affiliation
|
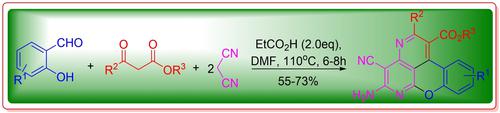
A novel approach to the synthesis of substituted 5-amino-4-cyanochromeno[4,3,2-de][1,6]naphthyridine-1-carboxylates from a wide range of substituted 2-hydroxybenzaldehydes with alkyl 3-oxo-3-substituted propanoates and malononitrile was investigated via propionic acid-promoted cascade Knoevenagel condensation/Michael addition/intramolecularly nucleophilic addition accompanied by oxidative aromatization. This procedure provided a highly efficient and facile route to functionalized chromenonaphthyridines from readily available substrates under mild reaction conditions.
中文翻译:

通过伪四组分反应高效合成铬诺[4,3,2-de][1,6]萘啶衍生物
从广泛的取代 2-羟基苯甲醛与烷基 3-oxo-3合成取代的 5-amino-4-cyanochromeno[4,3,2- de ][1,6] naphthyridine -1-carboxylates 的新方法通过丙酸促进的级联 Knoevenagel 缩合/迈克尔加成/分子内亲核加成伴随氧化芳构化研究 - 取代的丙酸酯和丙二腈。该过程提供了一种高效且简便的途径,可在温和的反应条件下从容易获得的底物获得官能化的色壬萘。
更新日期:2021-09-10
中文翻译:

通过伪四组分反应高效合成铬诺[4,3,2-de][1,6]萘啶衍生物
从广泛的取代 2-羟基苯甲醛与烷基 3-oxo-3合成取代的 5-amino-4-cyanochromeno[4,3,2- de ][1,6] naphthyridine -1-carboxylates 的新方法通过丙酸促进的级联 Knoevenagel 缩合/迈克尔加成/分子内亲核加成伴随氧化芳构化研究 - 取代的丙酸酯和丙二腈。该过程提供了一种高效且简便的途径,可在温和的反应条件下从容易获得的底物获得官能化的色壬萘。


























 京公网安备 11010802027423号
京公网安备 11010802027423号