Energy Storage Materials ( IF 20.4 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.ensm.2021.09.006 Bing Wu 1 , Jan Luxa 1 , Evgeniya Kovalska 1 , Marek Ivo 2 , Huanjuan Zhou 1 , Roman Malek 1 , Petr Marvan 1 , Shuangying Wei 1 , Liping Liao 1 , Zdenek Sofer 1
|
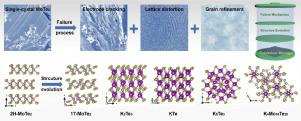
Potassium-ion batteries (KIBs) are competent candidates for next-generation energy-storage systems due to the source abundance, cost efficiency, and high energy density from comparable electrode potential to lithium. However, developing practical electrode materials for KIBs is still in its infancy, and the electrochemical reaction mechanisms for a specific material are far from clear. Here, MoTe2, due to the merits of sizeable ion-intercalation interlayer spacing of Van der Waals-type material, superior electron conductivity of its metal-semimetal characteristics to wildly studied MoS2, was for the first time investigated as the working electrode for potassium storage. The potassiation/depotassiation mechanisms were unravelled, combining electrochemical analysis, ex-situ scanning electron microscope (SEM), ex-situ transmission electron microscope (TEM) and in-situ X-ray diffraction. Sub-millimetre single-crystal MoTe2 displayed a high volumetric capacity of 792.4 mAh cm−3 at initial depotassiation and maintained a gravimetric specific capacity of 210 mAh g−1 at 100 mA g−1. It decayed rapidly after 25 cycles caused by the deactivation of active electrode material from the irreversible crystalline cracking and structure evolution during the electrochemical cycling. At initial potassiation, 2H-MoTe2 was irreversibly converted to 1T-MoTe2 and then further converted to potassium telluride. And at initial depotassiation over 2.5 V (vs. K/K+), a tentative K-Mo-Te compound with R-3H Cs4Mo18Te20 structure was formed and soon irreversibly converted to K2Te3 under following potassiation. Meanwhile, the stepwise reversible conversion of K2Te3-KTe-K5Te3 predominates the continuous electrochemical processes after the initial discharge/charge. Apart from the potential application for potassium-ion batteries, the conversion mechanisms amongst potassium tellurides also provide instructions for upcoming potassium-tellurium batteries.
中文翻译:

用于钾存储的亚毫米范德华单晶 MoTe2:电化学性质及其失效和结构演化机制
钾离子电池 (KIB) 是下一代储能系统的有力候选者,因为其来源丰富、成本效率高,并且从可比的电极电位到锂的高能量密度。然而,开发用于KIBs的实用电极材料仍处于起步阶段,具体材料的电化学反应机制尚不清楚。这里,MoTe 2,由于范德瓦尔斯型材料具有相当大的离子嵌入层间距的优点,其金属-半金属特性的优异电子传导性比广泛研究的MoS 2,首次被研究作为钾储存的工作电极。结合电化学分析、异位扫描电子显微镜 (SEM)、异位透射电子显微镜 (TEM) 和原位 X 射线衍射,揭示了钾化/脱钾机制。亚毫米单晶 MoTe 2在初始脱钾时显示出 792.4 mAh cm -3的高体积容量,并在 100 mA g - 1时保持了 210 mAh g - 1的重量比容量. 它在 25 次循环后迅速衰减,这是由于电化学循环过程中不可逆的晶体开裂和结构演变导致活性电极材料失活。在初始钾化时,2H-MoTe 2不可逆地转化为1T-MoTe 2,然后进一步转化为碲化钾。并且在超过 2.5 V (vs. K/ K + ) 的初始脱钾时,形成了具有 R-3H Cs 4 Mo 18 Te 20结构的暂定 K-Mo-Te 化合物,并在随后的钾化作用下很快不可逆地转化为 K 2 Te 3。同时,K 2 Te 3的逐步可逆转化-KTe-K 5 Te 3在初始放电/充电后主导连续电化学过程。除了钾离子电池的潜在应用外,碲化钾之间的转化机制也为即将推出的钾碲电池提供了指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号