当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interfacial Water Structure of Binary Liquid Mixtures Reflects Nonideal Behavior
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.jpcb.1c06001 Xiaoqing Yu 1 , Takakazu Seki 1 , Chun-Chieh Yu 1 , Kai Zhong 2 , Shumei Sun 3 , Masanari Okuno 4 , Ellen H G Backus 1, 5 , Johannes Hunger 1 , Mischa Bonn 1 , Yuki Nagata 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.jpcb.1c06001 Xiaoqing Yu 1 , Takakazu Seki 1 , Chun-Chieh Yu 1 , Kai Zhong 2 , Shumei Sun 3 , Masanari Okuno 4 , Ellen H G Backus 1, 5 , Johannes Hunger 1 , Mischa Bonn 1 , Yuki Nagata 1
Affiliation
|
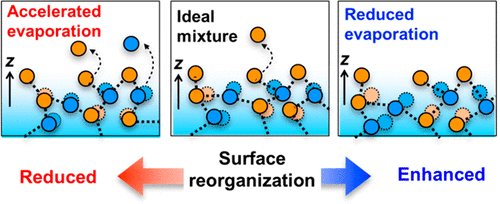
The evaporation of molecules from water–organic solute binary mixtures is key for both atmospheric and industrial processes such as aerosol formation and distillation. Deviations from ideal evaporation energetics can be assigned to intermolecular interactions in solution, yet evaporation occurs from the interface, and the poorly understood interfacial, rather than the bulk, structure of binary mixtures affects evaporation kinetics. Here we determine the interfacial structure of nonideal binary mixtures of water with methanol, ethanol, and formic acid, by combining surface-specific vibrational spectroscopy with molecular dynamics simulations. We find that the free, dangling OH groups at the interfaces of these differently behaving nonideal mixtures are essentially indistinguishable. In contrast, the ordering of hydrogen-bonded interfacial water molecules differs substantially at these three interfaces. Specifically, the interfacial water molecules become more disordered (ordered) in mixtures with methanol and ethanol (formic acid), showing higher (lower) vapor pressure than that predicted by Raoult’s law.
中文翻译:

二元液体混合物的界面水结构反映了非理想行为
水-有机溶质二元混合物中分子的蒸发对于大气和工业过程(如气溶胶形成和蒸馏)至关重要。与理想蒸发能量学的偏差可以归因于溶液中的分子间相互作用,但蒸发发生在界面上,而二元混合物的界面结构而不是整体结构影响蒸发动力学。在这里,我们通过将表面特定振动光谱与分子动力学模拟相结合,确定了水与甲醇、乙醇和甲酸的非理想二元混合物的界面结构。我们发现这些不同行为的非理想混合物界面处的自由悬空 OH 基团基本上无法区分。相比之下,氢键界面水分子的排序在这三个界面上大不相同。具体而言,界面水分子在与甲醇和乙醇(甲酸)的混合物中变得更加无序(有序),显示出比拉乌尔定律预测的更高(更低)的蒸气压。
更新日期:2021-09-23
中文翻译:

二元液体混合物的界面水结构反映了非理想行为
水-有机溶质二元混合物中分子的蒸发对于大气和工业过程(如气溶胶形成和蒸馏)至关重要。与理想蒸发能量学的偏差可以归因于溶液中的分子间相互作用,但蒸发发生在界面上,而二元混合物的界面结构而不是整体结构影响蒸发动力学。在这里,我们通过将表面特定振动光谱与分子动力学模拟相结合,确定了水与甲醇、乙醇和甲酸的非理想二元混合物的界面结构。我们发现这些不同行为的非理想混合物界面处的自由悬空 OH 基团基本上无法区分。相比之下,氢键界面水分子的排序在这三个界面上大不相同。具体而言,界面水分子在与甲醇和乙醇(甲酸)的混合物中变得更加无序(有序),显示出比拉乌尔定律预测的更高(更低)的蒸气压。



























 京公网安备 11010802027423号
京公网安备 11010802027423号