当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Folding in Place: Design of β-Strap Motifs to Stabilize the Folding of Hairpins with Long Loops
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.joc.1c01442 Alexis D Richaud 1 , Guangkuan Zhao 1 , Samir Hobloss 1 , Stéphane P Roche 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.joc.1c01442 Alexis D Richaud 1 , Guangkuan Zhao 1 , Samir Hobloss 1 , Stéphane P Roche 1
Affiliation
|
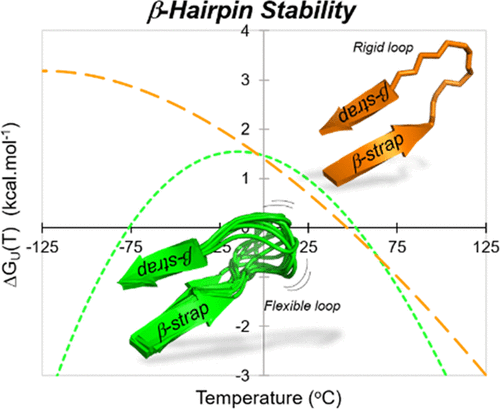
Despite their pivotal role in defining antibody affinity and protein function, β-hairpins harboring long noncanonical loops remain synthetically challenging because of the large entropic penalty associated with their conformational folding. Little is known about the contribution and impact of stabilizing motifs on the folding of β-hairpins with loops of variable length and plasticity. Here, we report a design of minimalist β-straps (strap = strand + cap) that offset the entropic cost of long-loop folding. The judicious positioning of noncovalent interactions (hydrophobic cluster and salt-bridge) within the novel 8-mer β-strap design RW(V/H)W···WVWE stabilizes hairpins with up to 10-residue loops of varying degrees of plasticity (Tm up to 52 °C; 88 ± 1% folded at 18 °C). This “hyper” thermostable β-strap outperforms the previous gold-standard technology of β-strand-β-cap (16-mer) and provides a foundation for producing new classes of long hairpins as a viable and practical alternative to macrocyclic peptides.
中文翻译:

原地折叠:设计 β 带图案以稳定长环发夹的折叠
尽管它们在定义抗体亲和力和蛋白质功能方面发挥着关键作用,但由于与它们的构象折叠相关的大量熵损失,含有长非规范环的 β-发夹仍然具有综合挑战性。关于稳定基序对具有可变长度和可塑性环的 β-发夹折叠的贡献和影响知之甚少。在这里,我们报告了一种极简 β 带(带 = 链 + 帽)的设计,它抵消了长环折叠的熵成本。非共价相互作用(疏水簇和盐桥)在新型 8 聚体 β 带设计 RW(V/H)W...WVWE 中的明智定位使发夹稳定,具有多达 10 个不同程度可塑性的残基环(Ť米高达 52°C;88 ± 1% 在 18 °C 下折叠)。这种“超”热稳定性 β-带优于之前的 β-链-β-帽(16 聚体)黄金标准技术,并为生产新类别的长发夹作为大环肽的可行且实用的替代品提供了基础。
更新日期:2021-10-01
中文翻译:

原地折叠:设计 β 带图案以稳定长环发夹的折叠
尽管它们在定义抗体亲和力和蛋白质功能方面发挥着关键作用,但由于与它们的构象折叠相关的大量熵损失,含有长非规范环的 β-发夹仍然具有综合挑战性。关于稳定基序对具有可变长度和可塑性环的 β-发夹折叠的贡献和影响知之甚少。在这里,我们报告了一种极简 β 带(带 = 链 + 帽)的设计,它抵消了长环折叠的熵成本。非共价相互作用(疏水簇和盐桥)在新型 8 聚体 β 带设计 RW(V/H)W...WVWE 中的明智定位使发夹稳定,具有多达 10 个不同程度可塑性的残基环(Ť米高达 52°C;88 ± 1% 在 18 °C 下折叠)。这种“超”热稳定性 β-带优于之前的 β-链-β-帽(16 聚体)黄金标准技术,并为生产新类别的长发夹作为大环肽的可行且实用的替代品提供了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号