当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Predicting the Fire and Explosion Properties of Early-Phase Active Pharmaceutical Ingredients
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.oprd.1c00197 Antony Janes 1 , Roy C. Flanagan 2 , Andrew Payne 1 , Chris Newlands 3
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.oprd.1c00197 Antony Janes 1 , Roy C. Flanagan 2 , Andrew Payne 1 , Chris Newlands 3
Affiliation
|
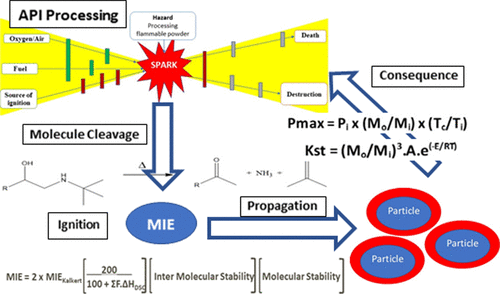
Early-phase drug product (DP) manufacture from an active pharmaceutical ingredient (API) is often performed in the absence of fire and explosion data, as material for testing either does not exist or cannot be spared. This leads to onerous processing restrictions, as properties are assumed to be “worst case” or processes are run with an inadequate basis of safety, leading to accidents. Full testing for fire and explosion properties uses about 800 g of material. This paper discusses predictive methodologies used by GSK R&D based on chemistry knowledge and experiments using <1 g of material to identify “materials of concern” (e.g., low minimum ignition energy or adverse dust explosion properties) that require additional safety measures or accelerated testing. The methodology has been employed on over 20 compounds in GSK R&D.
中文翻译:

预测早期活性药物成分的火灾和爆炸特性
使用活性药物成分 (API) 进行早期药物产品 (DP) 制造通常是在没有火灾和爆炸数据的情况下进行的,因为用于测试的材料不存在或无法幸免。这会导致繁重的处理限制,因为属性被假定为“最坏情况”,或者流程在安全基础不足的情况下运行,从而导致事故。防火和爆炸特性的全面测试使用约 800 克材料。本文讨论了 GSK R&D 基于化学知识和实验使用的预测方法,该方法使用 <1 g 的材料来识别需要额外安全措施或加速测试的“关注材料”(例如,低最小点火能量或不利的粉尘爆炸特性)。该方法已被用于 GSK 研发中的 20 多种化合物。
更新日期:2021-10-15
中文翻译:

预测早期活性药物成分的火灾和爆炸特性
使用活性药物成分 (API) 进行早期药物产品 (DP) 制造通常是在没有火灾和爆炸数据的情况下进行的,因为用于测试的材料不存在或无法幸免。这会导致繁重的处理限制,因为属性被假定为“最坏情况”,或者流程在安全基础不足的情况下运行,从而导致事故。防火和爆炸特性的全面测试使用约 800 克材料。本文讨论了 GSK R&D 基于化学知识和实验使用的预测方法,该方法使用 <1 g 的材料来识别需要额外安全措施或加速测试的“关注材料”(例如,低最小点火能量或不利的粉尘爆炸特性)。该方法已被用于 GSK 研发中的 20 多种化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号