Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiple Activation Catalyst for Asymmetric [4+2] Cycloaddition of Aldehydes with Dienes
Synlett ( IF 2 ) Pub Date : 2021-09-09 , DOI: 10.1055/s-0040-1720885 Takuya Kurahashi , Seijiro Matsubara , Rei Tomifuji , Shunpei Murano , Satoru Teranishi , Daiki Kuroda
中文翻译:

醛与二烯不对称 [4+2] 环加成反应的多重活化催化剂
更新日期:2021-09-10
Synlett ( IF 2 ) Pub Date : 2021-09-09 , DOI: 10.1055/s-0040-1720885 Takuya Kurahashi , Seijiro Matsubara , Rei Tomifuji , Shunpei Murano , Satoru Teranishi , Daiki Kuroda
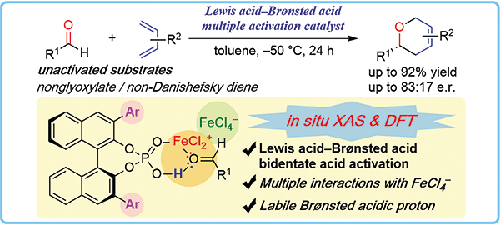
|
The enantioselective oxa-Diels–Alder reaction of nonactivated substrates by utilizing FeCl3 and a 1,1′-bi-2-naphthol (BINOL) derived chiral phosphoric acid as a multiple activation catalyst is reported. Various oxygen-containing six-membered heterocycles were obtained in high yields and in an enantioselective manner. Density functional theory (DFT) calculations elucidate that both Lewis acidic and Brønsted acidic moieties in the catalyst system synergistically activate two lone pairs of an aldehyde to facilitate enantioselective addition reaction of dienes.
中文翻译:

醛与二烯不对称 [4+2] 环加成反应的多重活化催化剂
报道了利用 FeCl 3和 1,1'-双-2-萘酚 (BINOL) 衍生的手性磷酸作为多重活化催化剂对未活化底物进行对映选择性 oxa-Diels-Alder 反应。以高产率和对映选择性方式获得了各种含氧六元杂环。密度泛函理论 (DFT) 计算表明,催化剂体系中的路易斯酸性和布朗斯台德酸性部分协同活化醛的两个孤对电子以促进二烯的对映选择性加成反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号