当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
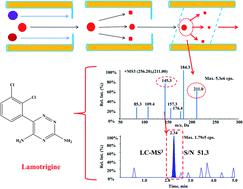
A LC-MS3 strategy to determine lamotrigine by Q-Q-trap tandem mass spectrometry coupled with triple stage fragmentation to enhance sensitivity and selectivity
Analytical Methods ( IF 3.1 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ay01372f Meiyun Shi 1 , Qiuhong Jiang 1 , Qiushi Lyu 1 , Zhengting Yuan 2 , Lili Deng 3, 4 , Lei Yin 1, 5
Analytical Methods ( IF 3.1 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1ay01372f Meiyun Shi 1 , Qiuhong Jiang 1 , Qiushi Lyu 1 , Zhengting Yuan 2 , Lili Deng 3, 4 , Lei Yin 1, 5
Affiliation
|
A high-performance liquid chromatography tandem mass spectrometry cubed (HPLC/MS3) method was developed and validated to quantify lamotrigine in human plasma with carbamazepine as an internal standard. The HPLC/MS/MS system is composed of a Shimadzu UFLC XR high-performance liquid chromatograph coupled with a hybrid linear ion trap triple quadrupole mass spectrometer. Following simple protein precipitation with methanol, the separation of lamotrigine and carbamazepine was performed on an Agilent Poroshell 120 SB-C18 column (4.6 × 50 mm, 2.7 μm) using gradient elution with 0.1% formic acid in water (solvent I) and 0.1% formic acid in methanol (solvent II) at a flow rate of 0.8 mL min−1. The total run time for each sample was 5 min. The method was validated for accuracy, precision, linearity, lower limit of quantification (LLOQ), selectivity, and other parameters. The LC/MS3 method was linear in the concentration range of 0.50–50.0 μg mL−1 (R2 ≥ 0.995). The LLOQ was 0.5 μg mL−1, requiring only 30 μL of human plasma. Intra- and inter-day accuracies were <6.17% and precisions were <11.4% at all concentrations. The absolute recoveries (%) and matrix effect (%) for lamotrigine in human plasma were between 83.8 and 90.7. The developed and validated LC-MS3 assay was successfully applied to monitor the lamotrigine levels in human plasma after the administration of lamotrigine.
中文翻译:

通过 QQ 阱串联质谱法测定拉莫三嗪的 LC-MS3 策略与三段碎裂相结合,以提高灵敏度和选择性
开发并验证了一种高效液相色谱串联质谱立方 (HPLC/MS 3 ) 方法,用于以卡马西平作为内标对人血浆中的拉莫三嗪进行定量。HPLC/MS/MS 系统由 Shimadzu UFLC XR 高性能液相色谱仪和混合线性离子阱三重四极杆质谱仪组成。在用甲醇进行简单的蛋白质沉淀后,在 Agilent Poroshell 120 SB-C18 色谱柱(4.6 × 50 mm,2.7 μm)上使用 0.1% 甲酸水溶液(溶剂 I)和 0.1% 的梯度洗脱进行拉莫三嗪和卡马西平的分离甲酸的甲醇溶液(溶剂 II),流速为 0.8 mL min -1. 每个样品的总运行时间为 5 分钟。该方法已经过准确度、精密度、线性、定量下限 (LLOQ)、选择性和其他参数的验证。LC/MS 3方法在0.50–50.0 μg mL -1 ( R 2 ≥ 0.995)的浓度范围内呈线性。LLOQ 为 0.5 μg mL -1,仅需要 30 μL 人血浆。在所有浓度下,日内和日间准确度<6.17%,精密度<11.4%。拉莫三嗪在人血浆中的绝对回收率 (%) 和基质效应 (%) 介于 83.8 和 90.7 之间。开发和验证的 LC-MS 3测定已成功应用于监测拉莫三嗪给药后人血浆中的拉莫三嗪水平。
更新日期:2021-09-10
中文翻译:

通过 QQ 阱串联质谱法测定拉莫三嗪的 LC-MS3 策略与三段碎裂相结合,以提高灵敏度和选择性
开发并验证了一种高效液相色谱串联质谱立方 (HPLC/MS 3 ) 方法,用于以卡马西平作为内标对人血浆中的拉莫三嗪进行定量。HPLC/MS/MS 系统由 Shimadzu UFLC XR 高性能液相色谱仪和混合线性离子阱三重四极杆质谱仪组成。在用甲醇进行简单的蛋白质沉淀后,在 Agilent Poroshell 120 SB-C18 色谱柱(4.6 × 50 mm,2.7 μm)上使用 0.1% 甲酸水溶液(溶剂 I)和 0.1% 的梯度洗脱进行拉莫三嗪和卡马西平的分离甲酸的甲醇溶液(溶剂 II),流速为 0.8 mL min -1. 每个样品的总运行时间为 5 分钟。该方法已经过准确度、精密度、线性、定量下限 (LLOQ)、选择性和其他参数的验证。LC/MS 3方法在0.50–50.0 μg mL -1 ( R 2 ≥ 0.995)的浓度范围内呈线性。LLOQ 为 0.5 μg mL -1,仅需要 30 μL 人血浆。在所有浓度下,日内和日间准确度<6.17%,精密度<11.4%。拉莫三嗪在人血浆中的绝对回收率 (%) 和基质效应 (%) 介于 83.8 和 90.7 之间。开发和验证的 LC-MS 3测定已成功应用于监测拉莫三嗪给药后人血浆中的拉莫三嗪水平。


























 京公网安备 11010802027423号
京公网安备 11010802027423号