当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanisms of Ssp3–H functionalization of thiolacetic acid: A density functional theory investigation
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-09-09 , DOI: 10.1002/poc.4279 Qian‐Mei Hou 1 , Da‐Gang Zhou 2
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-09-09 , DOI: 10.1002/poc.4279 Qian‐Mei Hou 1 , Da‐Gang Zhou 2
Affiliation
|
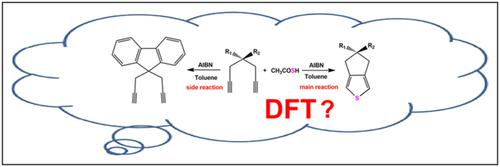
Mechanisms for Ssp3–H functionalization of thiolacetic acid, initiated by AIBN, have been investigated by M06-2X-D3/ma-def2-TZVP method and basis set, and SMD model was employed to simulate the solvent effect of toluene. The computational results suggest that azodiisobutyronitrile (AIBN), as the radical initiator, would have an intramolecular homolytic reaction; then Ssp3–H activation of thiolacetic acid could be finished via two possible paths; the obtained thiyl radical and diyne would go through electrophilic reaction, ring-closure reaction, and SN2 reaction to yield 3,4-fused-ring-substituted thiophenes. While in the second part, the mechanism of side reaction has also been investigated, revealing that main reaction has a lower energy barrier. Localized orbital locator isosurfaces and electron spin density isosurface graphs can analyze the structures and reveal the substances.
中文翻译:

硫羟乙酸的 Ssp3-H 官能化机制:密度泛函理论研究
通过M06-2X-D3/ma-def2-TZVP方法和基组研究了由AIBN引发的硫羟乙酸的Ssp 3 -H官能化机理,并采用SMD模型模拟了甲苯的溶剂效应。计算结果表明,偶氮二异丁腈(AIBN)作为自由基引发剂,会发生分子内均裂反应;然后 Ssp 3硫羟乙酸的-H活化可以通过两种可能的途径完成;得到的硫自由基与二炔经过亲电反应、闭环反应和SN2反应生成3,4-稠环取代的噻吩。而在第二部分中,也研究了副反应的机理,揭示了主反应的能垒较低。局部轨道定位器等值面和电子自旋密度等值面图可以分析结构并揭示物质。
更新日期:2021-09-09
中文翻译:

硫羟乙酸的 Ssp3-H 官能化机制:密度泛函理论研究
通过M06-2X-D3/ma-def2-TZVP方法和基组研究了由AIBN引发的硫羟乙酸的Ssp 3 -H官能化机理,并采用SMD模型模拟了甲苯的溶剂效应。计算结果表明,偶氮二异丁腈(AIBN)作为自由基引发剂,会发生分子内均裂反应;然后 Ssp 3硫羟乙酸的-H活化可以通过两种可能的途径完成;得到的硫自由基与二炔经过亲电反应、闭环反应和SN2反应生成3,4-稠环取代的噻吩。而在第二部分中,也研究了副反应的机理,揭示了主反应的能垒较低。局部轨道定位器等值面和电子自旋密度等值面图可以分析结构并揭示物质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号