Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.jece.2021.106358 Longqian Wang 1 , Jingya ye 1 , Jingyi Zhang 1 , Qinglong Meng 1 , Xiaoyu Li 1 , Zhonglin Chen 1 , Huahu Yu 1 , Ao Zhang 1 , Zhuoyu Bu 1 , Yongli Jiao 2 , Yuwei Pan 1
|
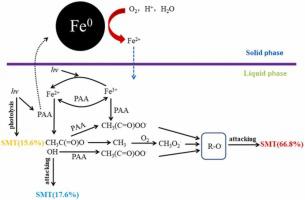
Peracetic acid (PAA) was successfully activated by UV and Fe0 to degrade sulfamethazine (SMT) in this study. UV/Fe0/PAA system not only could enhance SMT removal efficiency but also could reduce the energy consumption in comparison with UV, Fe0/PAA and UV/PAA systems. The R O• radical was the main radical for the degradation of SMT in UV/Fe0/PAA system and photolysis, •OH and R-O• exhibited 15.6%, 17.6% and 66.8% contribution for SMT degradation, respectively. Fourier transform infrared spectroscopy (FTIR), Scanning Electron Microscope (SEM), and X-ray photoelectron spectroscopy (XPS) were conducted to investigate the characterization of Fe0 after reaction in UV/Fe0/PAA system. The possible SMT degradation pathway was also proposed. Affecting factors such as Fe0 dosage (0–0.2 g L−1), PAA concentration (0–200 μM) and initial pH (3–9) were also studied. Cl−, NO3-, SO42- and HCO3- had a negligible adverse effect on SMT removal in this system indicating the good adaptability to inorganic ions. In addition, UV/Fe0/PAA system had the good recycle ability of Fe0 and showed a good performance in removing antibiotic in the nature fresh water. Therefore, this study notably improved the knowledge of PAA activated by Fe0 and UV and showed the potential applicability of UV/Fe0/PAA system in degrading antibiotics in nature fresh water.
O• radical was the main radical for the degradation of SMT in UV/Fe0/PAA system and photolysis, •OH and R-O• exhibited 15.6%, 17.6% and 66.8% contribution for SMT degradation, respectively. Fourier transform infrared spectroscopy (FTIR), Scanning Electron Microscope (SEM), and X-ray photoelectron spectroscopy (XPS) were conducted to investigate the characterization of Fe0 after reaction in UV/Fe0/PAA system. The possible SMT degradation pathway was also proposed. Affecting factors such as Fe0 dosage (0–0.2 g L−1), PAA concentration (0–200 μM) and initial pH (3–9) were also studied. Cl−, NO3-, SO42- and HCO3- had a negligible adverse effect on SMT removal in this system indicating the good adaptability to inorganic ions. In addition, UV/Fe0/PAA system had the good recycle ability of Fe0 and showed a good performance in removing antibiotic in the nature fresh water. Therefore, this study notably improved the knowledge of PAA activated by Fe0 and UV and showed the potential applicability of UV/Fe0/PAA system in degrading antibiotics in nature fresh water.
中文翻译:

Fe0 和 UV 活化过乙酸去除磺胺二甲嘧啶:效率和机理研究
在本研究中,过氧乙酸 (PAA) 被紫外线和 Fe 0成功激活以降解磺胺二甲嘧啶 (SMT)。与UV、Fe 0 /PAA和UV/PAA系统相比,UV/Fe 0 /PAA系统不仅可以提高SMT去除效率,还可以降低能耗。将R Ô •自由基是为SMT的在UV / Fe的降解主要自由基0 / PAA系统和光解,• OH和RO •表现出15.6%,17.6%和SMT降解分别66.8%的贡献。进行傅里叶变换红外光谱 (FTIR)、扫描电子显微镜 (SEM) 和 X 射线光电子能谱 (XPS) 以研究 Fe 的表征 0在UV / Fe的反应后0 / PAA系统。还提出了可能的 SMT 降解途径。还研究了影响因素,例如 Fe 0剂量(0–0.2 g L -1)、PAA 浓度(0–200 μM)和初始 pH(3–9)。Cl -、NO 3 -、SO 4 2-和HCO 3 -在该系统中对SMT去除的不利影响可以忽略不计,表明对无机离子具有良好的适应性。此外,UV/Fe 0 /PAA体系具有良好的Fe 0回收能力并在去除自然界淡水中的抗生素方面表现出良好的性能。因此,本研究显着提高了对Fe 0和UV激活PAA 的认识,并展示了UV/Fe 0 /PAA 系统在降解自然界淡水中抗生素的潜在适用性。
0在UV / Fe的反应后0 / PAA系统。还提出了可能的 SMT 降解途径。还研究了影响因素,例如 Fe 0剂量(0–0.2 g L -1)、PAA 浓度(0–200 μM)和初始 pH(3–9)。Cl -、NO 3 -、SO 4 2-和HCO 3 -在该系统中对SMT去除的不利影响可以忽略不计,表明对无机离子具有良好的适应性。此外,UV/Fe 0 /PAA体系具有良好的Fe 0回收能力并在去除自然界淡水中的抗生素方面表现出良好的性能。因此,本研究显着提高了对Fe 0和UV激活PAA 的认识,并展示了UV/Fe 0 /PAA 系统在降解自然界淡水中抗生素的潜在适用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号