Journal of King Saud University-Science ( IF 3.8 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.jksus.2021.101597 Ignacio Lizana 1, 2 , Julio Colmenares-Zerpa 1, 3 , Gina Pecchi 1, 2 , R.J. Chimentão 1 , Eduardo J. Delgado 1, 2
|
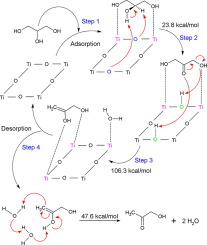
Glycerol is currently a co-product of biodiesel production, and it is well-known to be a platform molecule, which is widely used in etherification, dehydration, dehydrogenation, oxidation, and reforming reactions, to produce chemicals of high value for the chemical industry. In this study, we theoretically address the dehydration of glycerol over the SrTiO3-type perovskite. The study includes the characterization of the transition states and intermediates occurring along the reaction pathway, and a possible mechanism is proposed, as well. The results show that the dehydration of glycerol occurs via a four-stage mechanism corresponding to the adsorption of glycerol on the perovskite, the surface reaction to produce the adsorbed dihydroxyacetone, the elimination of water produce 2,3-enol, and finally the desorption of this enol, which in turn undergoes keto-enol tautomeric equilibrium to form hydroxyacetone (HA). The rate-limiting stage corresponds to the formation of 2,3-enol showing an activation barrier of 106.3 kcal/mol.
中文翻译:

在 SrTiO3 型钙钛矿上将甘油转化为羟基丙酮:DFT 研究
甘油是目前生物柴油生产的副产品,是众所周知的平台分子,广泛应用于醚化、脱水、脱氢、氧化、重整等反应,为化工生产高价值化学品. 在这项研究中,我们从理论上解决了甘油在 SrTiO 3 上的脱水问题。型钙钛矿。该研究包括对沿反应途径发生的过渡态和中间体的表征,并提出了可能的机制。结果表明,甘油的脱水通过四阶段机制发生,对应于甘油在钙钛矿上的吸附、表面反应生成吸附的二羟基丙酮、水的消除生成 2,3-烯醇,最后解吸这种烯醇依次经历酮-烯醇互变异构平衡以形成羟基丙酮(HA)。限速阶段对应于 2,3-烯醇的形成,显示出 106.3 kcal/mol 的活化势垒。


























 京公网安备 11010802027423号
京公网安备 11010802027423号