Journal of Structural Biology ( IF 3 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.jsb.2021.107795 Antonette Bennett 1 , Joshua Hull 1 , Nelly Jolinon 2 , Julie Tordo 3 , Katie Moss 1 , Enswert Binns 1 , Mario Mietzsch 1 , Cathleen Hagemann 4 , R Michael Linden 3 , Andrea Serio 4 , Paul Chipman 1 , Duncan Sousa 5 , Felix Broecker 6 , Peter Seeberger 7 , Els Henckaerts 8 , Robert McKenna 1 , Mavis Agbandje-McKenna 1
|
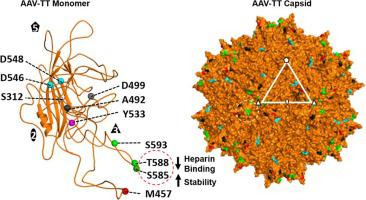
Adeno-associated viruses (AAV) are utilized as gene transfer vectors in the treatment of monogenic disorders. A variant, rationally engineered based on natural AAV2 isolates, designated AAV-True Type (AAV-TT), is highly neurotropic compared to wild type AAV2 in vivo, and vectors based on it, are currently being evaluated for central nervous system applications. AAV-TT differs from AAV2 by 14 amino acids, including R585S and R588T, two residues previously shown to be essential for heparan sulfate binding of AAV2. The capsid structures of AAV-TT and AAV2 visualized by cryo-electron microscopy at 3.4 and 3.0 Å resolution, respectively, highlighted structural perturbations at specific amino acid differences. Differential scanning fluorimetry (DSF) performed at different pH conditions demonstrated that the melting temperature (Tm) of AAV2 was consistently ∼5 °C lower than AAV-TT, but both showed maximal stability at pH 5.5, corresponding to the pH in the late endosome, proposed as required for VP1u externalization to facilitate endosomal escape. Reintroduction of arginines at positions 585 and 588 in AAV-TT caused a reduction in Tm, demonstrating that the lack of basic amino acids at these positions are associated with capsid stability. These results provide structural and thermal annotation of AAV2/AAV-TT residue differences, that account for divergent cell binding, transduction, antigenic reactivity, and transduction of permissive tissues between the two viruses. Specifically, these data indicate that AAV-TT may not utilize a glycan receptor mediated pathway to enter cells and may have lower antigenic properties as compared to AAV2.
中文翻译:

真型和野生型 AAV2 的比较结构、生物物理学和受体结合研究
腺相关病毒 (AAV) 在单基因疾病的治疗中用作基因转移载体。一种基于天然 AAV2 分离株合理设计的变体,称为 AAV-True Type (AAV-TT),与体内野生型 AAV2 相比具有高度神经营养性,以及基于它的载体,目前正在评估中枢神经系统的应用。AAV-TT 与 AAV2 的区别在于 14 个氨基酸,包括 R585S 和 R588T,这两个残基先前被证明对 AAV2 的硫酸乙酰肝素结合至关重要。AAV-TT 和 AAV2 的衣壳结构通过冷冻电子显微镜分别以 3.4 和 3.0 Å 分辨率可视化,突出了特定氨基酸差异的结构扰动。在不同 pH 条件下进行的差示扫描荧光法 (DSF) 表明熔解温度 (T m) 的 AAV2 始终比 AAV-TT 低约 5 °C,但两者都在 pH 5.5 时表现出最大稳定性,对应于晚期内体中的 pH,这是 VP1u 外化促进内体逃逸所需的建议。在 AAV-TT 的 585 和 588 位重新引入精氨酸导致Tm降低,表明这些位置缺乏碱性氨基酸与衣壳稳定性有关。这些结果提供了 AAV2/AAV-TT 残基差异的结构和热注释,这解释了两种病毒之间不同的细胞结合、转导、抗原反应性和允许组织的转导。具体而言,这些数据表明 AAV-TT 可能不利用聚糖受体介导的途径进入细胞,并且与 AAV2 相比可能具有较低的抗原特性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号